
Physical Properties Of Metal And Non - Metals
Metal And Non-metals of Class 10
| Table of Content |
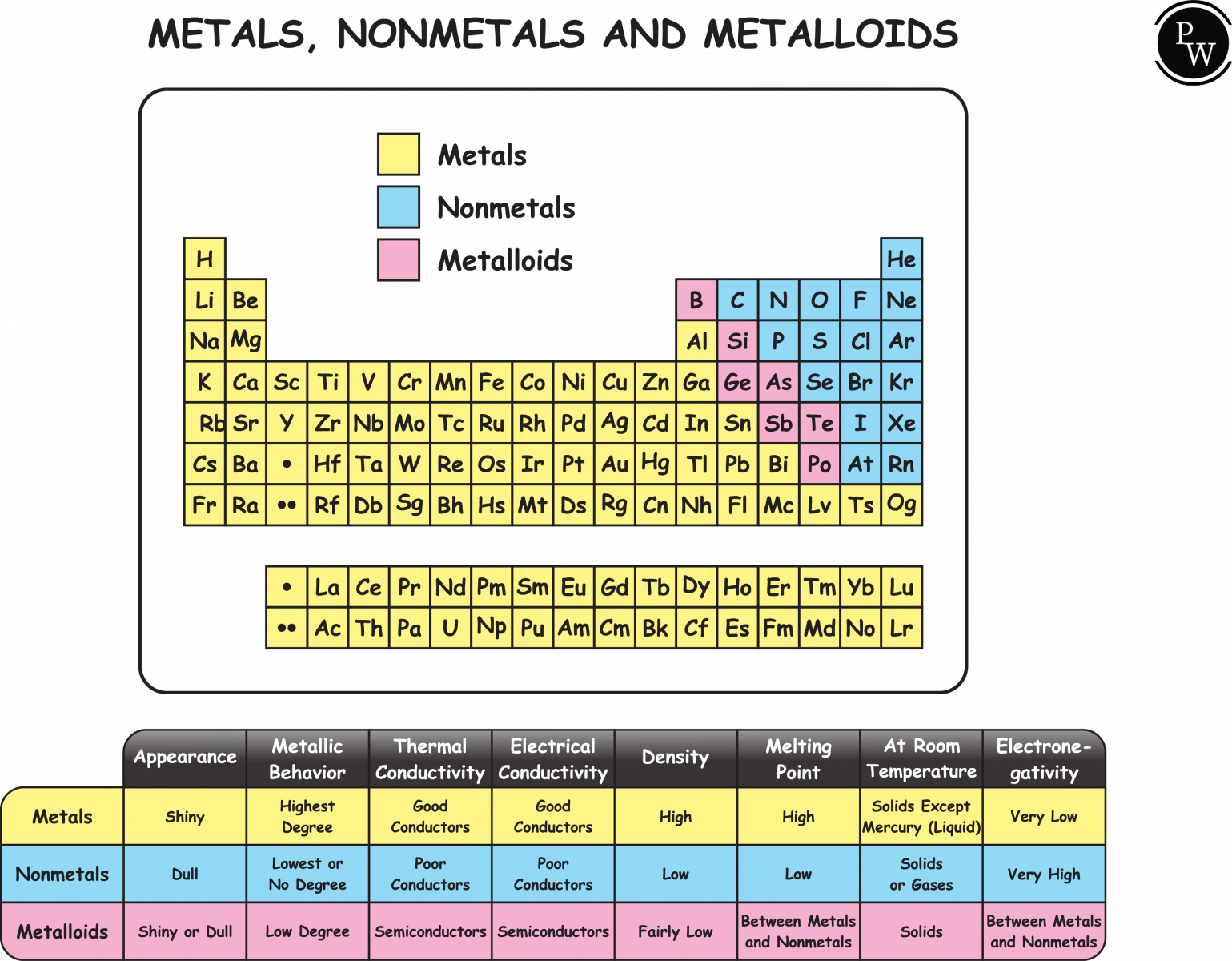
We will distinguish elements into metals and non-metals based on their chemical and physical properties. Metals have properties such as malleability, ductility, sound, and good conductors of heat and electricity. While non-metals are elements that are not malleable, ductile, or sound and are poor conductors of electricity and heat.
In this short article, we will discuss the properties of metals and non-metals and the difference between them based on their properties (with exceptions).
What is Metal?
In chemistry, a metal is defined as an element that can quickly form positive ions called cations and tends to form metallic bonds. Metals are characterized by their chemical and physical properties, such as malleability, ductility, ionization and bonding properties, etc.
Examples of metals include copper, silver, iron, mercury, lead, aluminum, gold, platinum, zinc, nickel, and tin.
Physical properties of Metal
Some physical properties of metals are mentioned below.
- Metals can be hammered into thin sheets. This means that they have the property of malleability.
- Metals are ductile. They can be drawn into wires.
- Metal are the best conductor of electricity and heat.
- Metals are lustrous, meaning they have a shiny appearance.
- Metals have high tensile strength. This means they can hold heavy weights.
- Metals are sonorous. This means that when we hit them, they make a ringing sound.
- Metals are hard. This means that they cannot be cut easily.
What is Non-Metal?
A non-metal in chemistry can be defined as a chemical element that generally gains electrons in a chemical reaction when it reacts with a metal. When combined with oxygen and hydrogen, it tends to form an acid. Nonmetals show a greater variety of colors and states compared to metals.
Examples of Non-metals are Hydrogen, chlorine, fluorine, carbon, nitrogen, phosphorus, and selenium.
Physical Properties of Non-Metal
- Non-metals are not malleable or brittle: Non-metals cannot be hammered or beaten into thin sheets without breaking. Non-metals break into pieces when hammered or stretched. Sulphur, phosphorous are powders and cannot be made into a sheet. Brittleness is a characteristic property of non-metals.
- Non-metals are not ductile: Non-metals cannot be melted and drawn into thin wires. Non-metals do not have free electrons. Thus the bonds between atoms in the elements are weak and they snap when stretched. The non-ductility property follows from the non-malleability or the brittleness property.
- Non-metals are bad conductors of heat and electricity: In non-metals, the bonds formed are weak as there are no free electrons to share. Other than graphite, which is an allotropic form of carbon, none of the non-metals are good conductors of heat and electricity. Graphite is able to conduct electricity because of its special crystalline arrangement.
- Non-metals have no lustre : Non-metals are in the form of powder or are gaseous. Hence they cannot be polished and they do not have any lustre. Most of the powders are dull in colour. Only graphite can be polished to some degree. Iodine shows some lustre as it has more electrons.
- Non-metals are not strong: Due to their non-ductile and non-malleable properties, non-metals are not strong at all. Their bonds break easily, as the electrons are not shared.
- Physical state: Non-metals may exist in solid, liquid or gaseous state at room temperature. For example sulphur and carbon are solid at room temperature, bromine is liquid and nitrogen, oxygen are gaseous non-metals.
- Melting and boiling points: All non-metals, have low melting and boiling points. The melting point of sulphur (S) is 115°C. Graphite and diamonds have high melting points, but these are exceptions in the non-metals.
- Solubility of non-metals : Non-metals are soluble in some chemical or organic solvents. For example iodine (I) is soluble in alcohol.
- Density of non-metals: Non-metals have low densities as compared to metals, which have high densities. This means that in non-metals, the atoms are not strongly bound. The crystalline volume of non-metals is small.
- Non-metals are not sonorous: Non-metals do not make any characteristic sound when hit with an object. Thus non-metals are not sonorous.
Chemical Properties of Metal and Non-Metal
Metallic elements usually have 1, 2, or 3 electrons in their outer shell. The smaller the number of valence electrons, the more active the metal. They form cations by losing electrons. The metal molecule in the vapor state is generally monoatomic. They generally form basic oxides. They ionize by losing electrons, that is why they are called reducing agents.
Non-metallic elements usually have 5, 6, or 7 electrons in their outer shell. They form anions (negative ions) by gaining electrons to complete their octet. Their molecules are mostly polyatomic in the gaseous state. They generally form acidic oxides. Nonmetals ionize by gaining electrons, which is why they are known as oxidizing agents.
Difference between Metal and Non-Metal
| Properties | Metals | Non-metals |
| State | Metals are solids at ordinary temperature except mercury,which is a liquid. | Non-metals exist in all the three states, that is, solid, liquid and gas. |
| Lustre | They possess lustre or shine. | They possess no luster except iodine and graphite. |
| Malleability and Ductility | Metals are generally malleable and ductile. | Non-metals are neither malleable nor ductile. |
| Hardness | Metals are generally hard. Alkali metals are exception. | Non-metals possess varying hardness. Diamond is an exception. It is the hardest substance known to occur in nature. |
| Density | They have high densities. | They generally possess low densities. |
| Conductivity | Metals are good conductors of heat and electricity. | Non-metals are poor conductors of heat are electricity. The only exception is graphite which is a good conductor of electricity. |
| Melting and boiling point | They usually have high melting and boiling point. | Their melting and boiling point are usually low. The exceptions are boron, carbon and silicon. |
Frequently Asked Question (FAQs)
Q1. How do Metals and Non-Metals differ chemically?
Ans. Metals generally do not combine with hydrogen. Some reactive metals combine with hydrogen to form ionic metal hydrides. Non-metals react with hydrogen to form stable, covalent hydrides.
Q2. How does metal react with water?
Ans. Metals react with water to form hydroxides or oxides and release Hydrogen gas.
Q3. Are non-metals soluble in water?
Ans. Non-metals react with oxygen to form oxides. Non-metal oxides are soluble in water. They dissolve in water to form acids. Metals generally react with acid to form salt and release hydrogen.
Q4. Which is the hardest non-metal?
Ans. Silicon Carbide is the hardest non-metal.
Q5. Which metal can float on water?
Ans. Sodium is that metal that can float on water.
Q6. Why are metals lustrous?
Ans. A metal is a lattice of metal "ions" in a "sea" of delocalised electrons - mobile electrons. The photons of light do not penetrate very far into the surface of a metal and are bounced off or typically reflected on the metallic surface by the mobile electrons, and what you get is metallic reflection, which is lustrous.









