
Mineral Salt Absorption
Mineral Nutrition In Plants of Class 11
Mineral Salt Absorption
- The necessity of mineral elements for plant growth has been proved beyond doubt. These are absorbed from the soil solution by the roots and are then translocated to various parts. Many theories have been given to explain the mechanism of mineral salt absorption. These theories can be grouped into following two categories:
- Passive absorption
- Active absorption
PASSIVE ABSORPTION
- Absorption of ions without the use of metabolic energy is known as passive absorption. Passive absorption may also be called physical absorption.
- It may be defined as the absorption of solute by cells, according to ordinary laws of diffuse Briggs and Robertson (1957) demonstrated the passive absorption of ions by root system in contact with soil colloids and root solution.
- They showed that the process is not affected by temperature and metabolic inhibitors and also that when a plant cell or tissue are transferred from a medium of low concentration to a medium of high concentration, there is a rapid uptake of ions.
- The direction of the initial uptake gets reversed if tissues are transferred back to low concentration.
- Based on these findings various theories have been proposed to explain the mechanism of salt uptake.
ION EXCHANGE THEORY
- According to this theory ions from the external solution are exchanged with the ions of similar charge adsorbed on the surface of the cell wall or membrane of the tissue proposed by Jenny and Overstreet (1938).
- The colloidal fraction of the soil (clay and humus) has an important role in ion exchange.
- These colloidal particles called micelles; posses negative charge and attract cations such as calcium, magnesium, potassium, ammonium, sodium as well as hydrogen ions arising from biological activity.
- Similarly, the root surface, which assumes a negative charge, carries many cations on its surface.
- These cations can be exchanged with other cations present in the soil. Thus, a new cation can be adsorbed on the root surface.
- Further transport of cations inside the root cell takes place by diffusion.
-
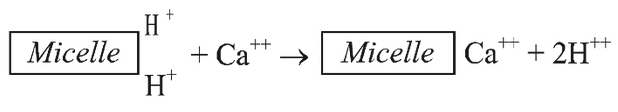
- This process does not involve participation of the metabolic energy. Like cations, anions can also be exchanged with free OH– ions.
MASS FLOW THEORY (BULK FLOW)
- According to Stylmo (1953, 1955) the ions are taken up by the roots along with mass flow of water under the influence of transpiration.
- This theory was supported by Kramer (1956) and Russel and Barber (1960).
- Later Lopushinsky (1964) worked on this problem and studied the uptake of radioactive P32 and Ca45.
- He found that an increase in the hydrostatic pressure (comparable to transpiration pull) increases ion uptake. So transpiration effect on salt absorption is direct.
DONNAN EQUILIBRIUM
- This mechanism, given by F.G. Donnan (1927) takes into the effect on non diffusible ions, which may be present on one side of the membrane. Unlike diffusible ions, the membrane is not permeable to non diffusible ions. Such ions are termed as fixed ions. They may be anions or cations. In a system, in which there are no fixed ions, there are equal number of anions and cations on both sides of the membranes at equilibrium. But in Donnan equilibrium, in order to balance the charge of fixed ions (e.g., anions) more ions of other charge (e.g., cations) would be required mathematically, the Donnan equilibrium may be represent by the following equation:
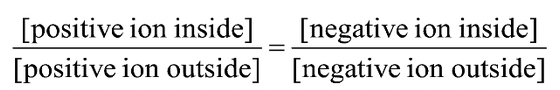
ACTIVE ABSORPTION
- Generally, the lipid-protein membrane of a cell is largely permeable to free ions.
- The energy is considered to be involved in the transport of such free ions across the membrane. The absorption of ions involving use of metabolic energy is called active absorption.
- Energy used in these mechanisms comes from metabolic activities especially respiration.
- Carrier concept: This theory of active absorption suggests involvement of definite chemical compound, called carrier, present in the membrane. The membrane does not allow the ions to pass through as it is. The ions form a complex with the carrier called carrier-ion complex, which is capable of moving across the membrane. The ions are released on the inner side of the membrane.
- The carriers are specific i.e., they combine with a particular type of ion. Carriers are probably proteinaceous in nature because addition of protein degrading substances in the system also reduces the rate of adsorption.
- Anion respiration hypothesis: This theory was proposed by Lundegardh (1954). According to this explanation only anions are absorbed actively, i.e., anion uptake requires energy, (i.e., they are absorbed actively).
- At the outer surface of the membrane, the cytochrome undergoes oxidation and loses one electron and in exchange picks up an anion.
- This is then transported to the inner side of the membrane through the cytochrome chain and on the inner surface of the membrane, the anion is released and the cytochrome gets reduced by the action of dehydrogenase involved in respiration.
- The cations move passively along the electrical gradient created by the accumulation of anions at the inner surface of the membrane.
The evidence in favour of Lundegardh’s hypothesis is that the respiration is increased when a plant is transferred from water to salt solution. The increased respiration was called salt respiration or anion respiration.
FACTORS AFFECTING SALT ABSORPTION
- Several factors affect salt absorption. Some of these are as follows:
TEMPERATURE
- Though within a narrow range, the increase in temperature increases both passive and active salt absorption processes and a lowering in temperature decrease them.
- This is because the same relationship exists between temperature and free diffusion involved in passive absorption and temperature and biochemical relations involved in active salt absorption. Beyond a certain maximum limits of temperature salt absorption gets inhibited and stops because of denaturation of enzymes involved directly or indirectly in the process.
- pH
- As the pH affects the availability of ions in the medium, it may directly affect absorption also. When sufficient ions are present in the medium, the effects of pH over a physiological range on salt absorption are not prominent.
- However, at pH values out side the physiological range damage the plant tissue and carriers inhibit salt absorption.
LIGHT
- As stomata when open allow more transpiration and increased mass flow and photosynthesis provides energy and oxygen for salt uptake, light indirectly affects the rate of salt absorption by affecting the opening and closing of stomata and the process of photosynthesis.
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2025 Physicswallah Limited All rights reserved.
Get App








