
A Liquid solution is a homogeneous mixture of two or more substances in which the solute (component present in the lesser quantity) is uniformly dispersed in the solvent (component present in the larger quantity). Components in solutions are uniformly distributed and solute particles dissolve completely in solvent particles.
Types of Solutions
Gaseous Solutions:
Both solute and solvent are gases.
E.g., air. Liquid Solutions: Solute can be a gas, liquid, or solid; solvent is liquid. E.g., salt in water, oxygen in water.
Solid Solutions:
Solute can be a gas, liquid, or solid; solvent is solid.
E.g., alloys like bronze (copper and tin).
Expressing Concentration of Solutions
Mass Percentage (w/w):
![]()
Volume Percentage (v/v):
![]()
Mass by Volume Percentage (w/v):
![]()
Parts per million (PPM):
![]()
Parts per billion (PPB):
![]()
Molarity (M):
![]()
Molality (m):
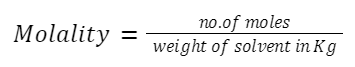
Normality (N)
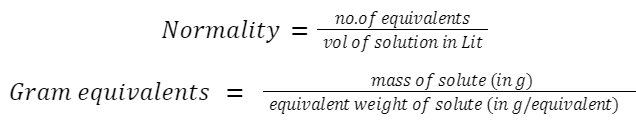
Mole Fraction:
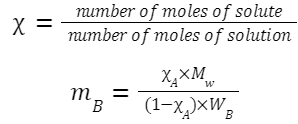
m B = molality of component B
X A = mole fraction of component A
M W = molar mass of the solvent (in g/mol)
W B = molar mass of the solute (in g/mol)
Relation between Molarity (M) and Molality (m)
ρ = density of the solution (in g/L)
M₁ = molar mass of the solute (in g/mol) M₂ = molar mass of the solvent (in g/mol)
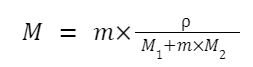
Relation between Molarity (M) and Mole Fraction (χ)
For a binary solution (solute A and solvent B):
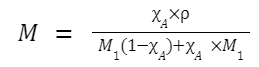
Relation between Normality (N) and Molarity (M)
For a substance that can furnish n equivalents per molecule (like H₂SO₄ which can furnish 2 moles of H⁺ ions):
N = n×M
Relation between Molality (m) and Mole Fraction (χ)
For a binary solution:
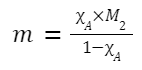
Relation between Molarity (M), Normality (N), and Molality (m)
Considering that:
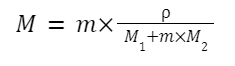
Also Check – Value of Gas Constant Formula
Henry's Law
According to Henry's Law, the partial pressure of a gas above a solution (P) is exactly proportional to the solubility (S) of that gas at a given temperature.
S = k H × P
where k H is Henry's law constant.
Mole fraction of gas in the solution
P = k H ×Mole fraction of gas in the solution
The value of k H is specific for each gas and changes with temperature.
Also Read - Acetone Formula
Vapour Pressure
The vapour pressure of a solution is the pressure exerted by its vapor when the solution is in dynamic equilibrium with its vapor in a closed system. For a solution, the presence of a non-volatile solute decreases its vapour pressure.
Colligative Properties
Colligative properties are those of solutions that depend on the quantity of those particles.
Relative Lowering of Vapour Pressure
If P 0 is the vapour pressure of the pure solvent and P s is the vapour pressure of the solution,
Mole fraction of solute
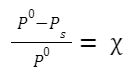
Elevation in Boiling Point
The boiling point of a solution is higher than that of the pure solvent.
ΔT b = K b ×m
Where: ΔT b = Elevation in boiling point
Kb = Ebullioscopic constant (specific to each solvent)
m = Molality of the solution
Depression in Freezing Point
The freezing point of a solution is lower than that of the pure solvent.
ΔT f =K f ×m
where: ΔT f = Depression in freezing point
K f = Cryoscopic constant (specific to each solvent)
Osmotic Pressure
It is the pressure required to prevent the flow of solvent into the solution through a semi-permeable membrane.
π = CRT
Where: π = Osmotic pressure
C = Molarity of the solution
R = Universal gas constant
T = Absolute temperature (Kelvin)
Also Check – Ascorbic Acid Formula
Van't Hoff Factor (i)
The van't Hoff factor (i) represents the number of ions a substance dissociates into when dissolved.
For non-electrolytes (like glucose), i=1. For electrolytes, i is greater than 1. For instance, for NaCl, which dissociates into Na + and Cl - , i=2.
Formula: i=1+α(n−1)
where: α = Degree of dissociation
n = Number of ions the solute dissociates into
RLVP:
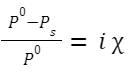
Elevation in boiling point: ΔT b = i× K b ×m
Depression in freezing point: ΔT f = i× K f ×m
Osmotic pressure: π = iCRT
Azeotropic Mixtures
Azeotropic mixtures are solutions with a vapor phase with the same composition as the liquid phase, preventing separation by distillation.
Positive azeotropes have a lower boiling point, containing higher vapor pressure components, while negative azeotropes have a higher boiling point and lower vapor pressure components. Examples include ethanol and water.
The van't Hoff factor (i) can be related to the observed molar mass (M obs ) and theoretical molar mass (M theo ) when we're considering the colligative properties influenced by dissociation or association in solution.
For a solute that dissociates:
For example, if a solute dissociates into n particles:
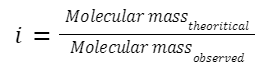
Liquid Solutions Formula FAQs
Q1. Define solubility.
Q2. What is a saturated solution?
Q3. What is an unsaturated solution?
Q4. How does temperature generally affect solubility of solids in liquids?
Q5. How does pressure affect the solubility of gases in liquids?










