
Acetone formula - Acetone is an extremely flammable organic compound. It has a chemical formula of C 3 H 6 O , and is also referred to as propanone. This colourless and volatile solvent can be found in auto exhausts, plants, trees, forest fires, urine and blood. It also mixes with several liquids, including water, ether and ethanol; and it has a pungent, floral or irritating odour. Acetone is widely used for antiseptic and solvent purposes. Alchemists carried out the first production of acetone through the dry distillation of metal acetates; while nowadays it is often derived from propylene via either direct or indirect methods. Most of the acetone available today is generated during the cumene process (83%). In addition to this method various other techniques can be used to produce it.
Structure of Acetone – C 3 H 6 O
One molecule of Acetone contains three carbon atoms, six hydrogen atoms, and one oxygen atom. Acetone is given the chemical formula as C3H6O. Acetone is also written as (CH3)2CO. The structure of these atoms in acetone is characterized by two methyl groups (CH3) attached on either side of a central carbon atom. In addition to being double-bonded with an oxygen atom, the middle carbon atom forms a carbonyl or ketone functional group.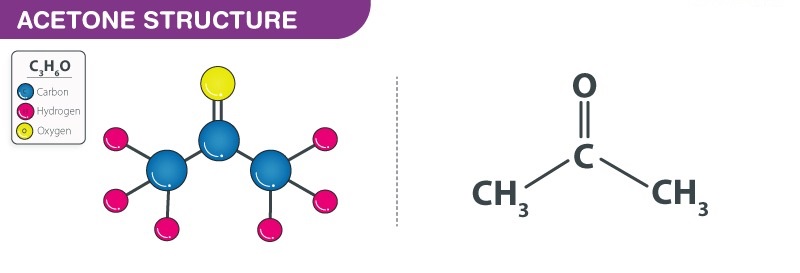
Properties of Acetone
| Properties of Acetone | |
| Chemical Formula | C 3 H 6 O |
| Molecular Weight/ Molar Mass | 58.08 g/mol |
| Boiling Point | 56℃ |
| Melting Point | -94.7 ℃ |
| Density | 0.7845 g/cm 3 |
| Appearance | Colorless Liquid |
Preparation of acetone
Cumene is used to produce 83% of acetone in the industry. Cumene is produced by alkylating benzene with propylene. The cumene is then oxidized by air to produce phenol and acetone. The phenol and acetone are then separated by distillation.Also Read: Malic Acid Formula
Chemical Properties of Acetone
Acetone belongs to the family of organic compounds called Ketones. It undergoes several chemical reactions because of its structural properties. Keto-enol Tautomerism Unlike most ketones, acetone has keto-enol tautomerism, which means that the enol isomer (CH3)C(OH)=(CH2) is in equilibrium with the nominal keto structure (CH3)2C=O. At ambient temperature, just 2.4107% of the molecules in acetone vapor are in the enol form. An important chemical reaction requires the presence of the enol form.Also Check - Nucleophile Formula
Haloform Reaction In the presence of alkali, acetone undergoes a haloform reaction when reacting with the CH3-C=O group.Uses of Acetone
- Acetone is a versatile chemical, often utilized in many industrial processes such as cleaning, chemical research, and carrying acetylene.
- It is employed in small doses to create products that break down or dissolve substances like nail polish, paint, and varnish.
- It can also be used for removing grease or gum from materials like wool and silk, as well as making lacquers for cars and furniture and plastics.
- Besides these uses, Acetone is also a precursor for methyl methacrylate which is found in certain drugs supplied by the pharmaceutical industry.
- Additionally, it serves to rinse lab glassware in laboratories.
Also Check - Tyndall Effect Formula
Health Hazards of acetone – C3H6O
- While highly flammable, acetone has low acute and chronic toxicity. If inhaled, acetone can cause sore throats and coughs.
- It is possible to irritate your nose, throat, lungs, and eyes by breathing moderate to high levels of acetone for a short period of time.
- There can also be headaches, dizziness, confusion, a faster pulse, nausea, vomiting, effects on the blood, passing out and possible coma, and a shorter menstrual cycle in women.
Also Check - Modern Periodic Table Formula
Things to remember
- Paint and grease can be dissolved or broken down by acetone, a colorless liquid solvent.
- The most important aliphatic ketone is acetone.
- Its other names are dimethyl ketone, 2-propanone, propanone, and beta-ketopropane.
- Pure acetone is an aromatic and flammable organic compound.
- Trees, tobacco smoke, vehicle exhaust, and landfills naturally contain it.
- Artificial fibers and explosives are manufactured with acetone.
Acetone Formula FAQs
What is acetone, and where is it commonly found?
Is acetone safe for skin contact?
Can acetone be used as a nail polish remover?
Is acetone flammable, and what precautions should be taken when using it?
Can acetone be used for cleaning purposes other than nail polish removal?










