

In the late 18th century, Mendeleev created a periodic table due to his discovery of the periodic law. This was before scientists understood the internal structure of atoms. As atomic models and quantum theory developed, it became clear that the atomic number is the primary characteristic of a chemical element. This shift from Mendeleev's law to what we now know as the modern periodic law reflects this progress.
History of the Periodic Table
The periodic table that we study today is the modern periodic table and was invented by Dmitri Mendeleev. However, Mendeleev was not the first one who grouped and arranged the elements in a periodic table. Prior to the recognition of the modern periodic table, the following attempts to classify elements occurred.- It was Antoine Lavoisier who classified elements based on their properties in 1789, dividing them into gases, non-metals, metals, and earthly elements.
- In 1829 Johann Döbereiner tried to classify elements according to their chemical properties into triads, where the middle element's atomic weight was usually the average of the first and third. As an example, Lithium, sodium and potassium. However, he was not able to group all known elements accurately, so the law of triads was not accepted.
- The attempt to classify elements did not stop here and the English Chemist John Alexander Newland arranged the elements in order of increasing atomic weights in 1865. He discovered a periodic pattern in the arrangement. He showed that the physical and chemical properties of the eighth element are similar to the properties of the first element in that row. This generalization is known as the 'Law of Octaves'.
- Mendeleev published a paper in 1869, in the Journal of Russian Chemical Society, which outlined the periodic law - that element properties are periodic functions of their atomic masses. He was able to classify 63 known elements into a table with 8 columns and 7 rows, and even had space for additional elements to be discovered later.
Modern Periodic Law Definition
Based on the modern Periodic law, it has been reported that the physical and chemical traits of elements are determined by their atomic numbers. This knowledge of elementary particles and quantum numbers provided scientists with information about electron configuration in the Periodic table. Thus, understanding this periodic law allowed chemists to analyze the correlation between 94 found elements. As a result of this analysis, chemists began producing various artificial elements which necessitated the reformation of Mendeleev’s periodic table as per modern periodic law.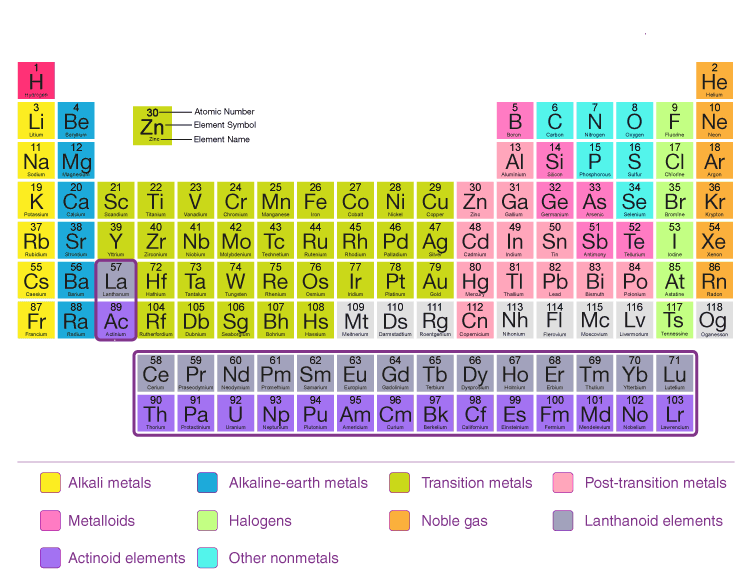
Features of Modern Periodic Table
Following are some of the main features of the modern periodic table in the study of chemistry.- The elements are arranged in ascending order of their atomic numbers.
- Periods are seven horizontal rows and groups are eighteen vertical columns
- In a group, elements have the same number of outer electrons, but as we move from top to bottom, their physical and chemical properties gradually change.
- As we move from left to right, the properties of the elements gradually change. Atomic size gradually decreases.
- As compared to Mendeleev's periodic table, the modern periodic table contains more elements.
- Without the modern periodic table, it would have been impossible to study the chemistry of elements.
- It is easier to understand the properties of elements when they are classified in the modern periodic table.\
Also Check - Aluminium Nitrate Formula
Classification of Elements in the Periodic Table
Alkali and Alkaline Earth Metal: A lot of highly reactive elements (except hydrogen) make up the alkali and alkaline earth metals on the left side of the periodic table. A first-group element has one electron in its valence shell, while a second-group element has two electrons. Transition Elements: Most of the properties of metals can be seen in transition metals, which are located at the center of the periodic table. Transition metals are composed of elements ranging from groups 3 to 12. Some transition metals are placed in two rows at the bottom of the periodic table as Lanthanides and Actinides. Mettaloids: At the right side of the periodic table are metaloids, which appear in a diagonal pattern. These elements are essentially the elements that separate metals on the left side of the periodic table from nonmetals on the right side. As they have the properties of both metals and nonmetals, metalloids are referred to as metalloids. Noble gas : These gases belong to the 18th group of the periodic table and have completely filled valence shells. They are non-reactive and are often called noble gases.Also Check - Ammonium Nitrate Formula
Modern Periodic Table Features & Significance
The modern periodic table contains elements we still use today. It is primarily based on Mendeleev's periodic table, but it also has some differences. As with previous tables, the elements here are arranged by increasing atomic number, not atomic mass. Here are some of the things we need to know:- According to their atomic numbers, the constituents of the table are arranged in increasing order.
- There are 7 horizontal rows and 18 vertical columns in the table, referred to as the rows.
- Each element in the table has the same physical and chemical properties. The outer electrons have the same number, but gradually change from the top to the bottom.
- When we move from left to right, the elements in the period change gradually. Atomic size decreases as well.
- Compared to Mendeleev's periodic table, this periodic table has about 118 elements.
Also Check - Elevation of Boiling Point Formula
Position of Elements in the Modern Periodic Table
In 1869, 63 atoms were discovered. Mendeleev classified them according to their atomic mass in the form of columns and rows known as his periodic table. This provided the basis for the modern version, which is based on increasing atomic number. To explain why, we must look into how each element’s characteristics are determined; atomic mass is determined by protons and neutrons while atomic number relies only on the number of electrons. Therefore, atoms are now arranged according to increasing electron count when placed in the modern periodic table.Explain the Position of Hydrogen in the Periodic Table
Mendeleev developed the Periodic Table of Elements based on increasing atomic mass and chemical properties, replacing Newland's Octave Law. This table organizes 118 atoms into 7 periods and 18 groups, with chemical properties changing from metal to metalloid to non-metal as you move across the period and the same throughout the group. Hydrogen is unique due to its properties of both metal and non-metal, not having a fixed location in the table. Lastly, two special periods are placed away from the main table because of the radioactive atoms found there - Lanthanides and Actinides.Modern Periodic Table Formula FAQs
What is the Modern Periodic Table?
The Modern Periodic Table is a tabular arrangement of chemical elements based on their atomic number, which reflects the number of protons in an atom's nucleus.
Why is the Modern Periodic Table an essential chemistry tool?
It provides a systematic framework for understanding element properties, predicting chemical behavior, and organizing the elements according to their atomic structure.
What information does the Modern Periodic Table provide about elements?
It offers information such as atomic number, symbol, atomic mass, and element name, helping scientists and students access key data about each element at a glance.
How are elements arranged in the Modern Periodic Table?
Elements are arranged in rows called periods and columns called groups, with similar chemical properties found in the same group.
How many elements are there in the Modern Periodic Table?
There are 118 elements in Modern Periodic Table.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2025 Physicswallah Limited All rights reserved.











