
The ammonium nitrate formula is NH4NO3. It consists of the ammonium cation (NH4)+ and the nitrate anion (NO3)–.
The fertiliser Ammonium Nitrate contains half nitrogen (N) in the nitrate form and half in the ammonium form, making it a widely used fertilizer. By using soil moisture to transport the nitrate form to the roots, it is instantly available to the plants for absorption.Also Read - Aluminum Acetate Formula
Structure of Ammonium Nitrate
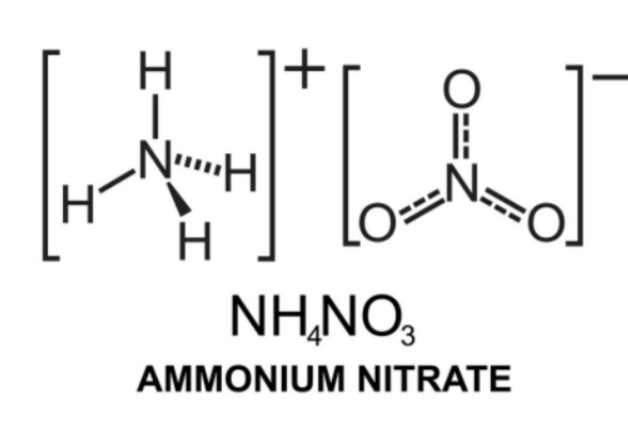 This compound has an ionic bond between an ammonium ion and a nitrate ion. This structure is illustrated below. Ammonium Nitrate Structure The pi electrons in the nitrate ion are delocalized through resonance. As the nitrogen atom holds a charge of +1 and each oxygen atom holds a charge of -23, the net charge of this ion is -1. Only one NH4+ ion can form an ionic bond with one NO3– ion.
This compound has an ionic bond between an ammonium ion and a nitrate ion. This structure is illustrated below. Ammonium Nitrate Structure The pi electrons in the nitrate ion are delocalized through resonance. As the nitrogen atom holds a charge of +1 and each oxygen atom holds a charge of -23, the net charge of this ion is -1. Only one NH4+ ion can form an ionic bond with one NO3– ion.
Chemical Data
| Chemical Formula | NH4NO3 |
| Molar Mass/ Molecular Weight | 80.043 grams per mole |
| Density | 1.725 grams per cubic centimetre |
| Melting Point | 442.8K (169.6oC) |
| Boiling Point | Decomposes at 483K (210oC) |
Production of Ammonium Nitrate Formula
As a result of an acid-base reaction between ammonia and nitric acid, NH4NO3 is produced. NH3 + HNO3 → NH4NO3 This reaction is very violent and exothermic. Ammonium nitrate is also well known for its oxidizing properties. In the mining and construction industries, it is used in many explosives. A major component of ANFO is NH4NO3, a widely used industrial explosive.Physical Properties of Ammonium Nitrate Formula
- The crystalline solid ammonium nitrate has a white/grey color and has a trigonal crystal structure.
- It is highly soluble in water at 200C.
- When the temperature is elevated to 100, the solubility increases to 1024g/100ml.
- The breakdown of NH4NO3 in H2O produces a lot of heat.
- The shock and friction sensitivities of this compound are extremely low.
Also Check - Aluminium Nitrate Formula
Chemical Properties of Ammonium Nitrate Formula
- Alkali metal nitrates and ammonia are produced when NH4NO3 reacts with alkali metal hydroxides.
- This chemical decomposes into nitrous oxide (N2O) and water when heated.
- When this chemical bursts, it produces N2, O2, and water as byproducts.
- Despite being part of many explosives, ammonium nitrate is not explosive.
- A primary explosive like azide must be added to it in order to create an explosive mixture. Due to its high nitrogen content of 34%, NH4NO3 forms a key component of many fertilisers, unlike urea which is prone to nitrogen loss into the atmosphere.When mixed with fuel oil, it produces ANFO - one of the most popular industrial explosives.Its endothermic dissolution in water makes it an ideal agent for quick cold packs.Furthermore, it is an ingredient in explosives utilized in both mining and construction industries.
Health Hazards of Ammonium Nitrate
- It is possible to find health and safety information in material safety data sheets available from suppliers and on the Internet.
- Ammonium nitrate, a non-toxic compound commonly used in fertilizer products, has an LD50 of 2217 mg/kg, roughly two-thirds that of table salt.
- When heated, ammonium nitrate breaks down non-violently into nitrous oxide and water vapor. Yet detonation can cause it to explode.
- Oxidation of big stores of the agent may additionally be an issue, since it can get out of hand and culminate in a blast quickly.
Also Check - Mole Fraction Formula
Ammonium Nitrate as Fertilizers
Ammonium nitrate is a highly sought-after fertilizer due to its components of both nitrogen-containing NH4 (ammonium) and NO3 (nitrate) molecules. Plants can absorb nitrogen directly from the nitrate form, and soil microorganisms can transform the ammonium part into nitrate. This makes it an ideal choice for vegetable farmers seeking a dependable nitrate source. Additionally, it is more stable than urea-based fertilizers, meaning that animal farmers can use it to provide nutrients in hay and pasture without the risk of volatilization. Furthermore, its solubility makes it a great option for irrigation systems as well.Applications of Ammonium Nitrate
- Due to its high nitrogen content of 34%, NH4NO3 forms a key component of many fertilizers, unlike urea which is prone to nitrogen loss into the atmosphere.
- When mixed with fuel oil, it produces ANFO - one of the most popular industrial explosives.
- Its endothermic dissolution in water makes it an ideal agent for quick cold packs.
- Furthermore, it is an ingredient in explosives utilized in both mining and construction industries.
- NH4NO3 is an ionic salt with many applications, the most noteworthy being its incorporation into fertilizers.
- It contains high nitrogen levels (34%) and does not readily dissipate as urea does.
- Commonly used to produce ANFO (an explosive created by mixing it with fuel oil), NH4NO3 is also a component in explosives employed in mining and construction.
- Additionally, this compound can be found in instant cold packs because of its endothermic character when dissolved in water.
- Even so, due to the potential for abuse, it is gradually becoming restricted by governments around the world – most notably seen in the 2011 Delhi bombings and 2013 Hyderabad explosions which both involved ammonium nitrate components.
Ammonium Nitrate Formula FAQs
What is ammonium nitrate?
Ammonium nitrate is a chemical compound with the formula NH4NO3, commonly used as a fertilizer and explosive.
Is ammonium nitrate safe to store at home?
Ammonium nitrate should not be stored at home due to its potential for explosive reactions.
How is ammonium nitrate used in agriculture?
It is used as a nitrogen fertilizer to provide essential nutrients to plants for growth.
Can ammonium nitrate be used in homemade explosives?
Yes, ammonium nitrate can be used in homemade explosives if combined with other substances, making it a security concern in some cases.
What safety precautions should be taken when handling ammonium nitrate?
Safety precautions include storing it away from heat and flammable materials, avoiding contamination, and following proper handling procedures.
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









