
Molybdic Acid Formula: Molybdenum is a chemical element on the periodic table, represented by the symbol Mo and atomic number 42. It belongs to period 5 and is part of group 6. This element was first identified in 1778 by Carl Wilhelm Scheele and isolated in 1781 by Peter Jacob Hjelm. Molybdenum is not found freely in nature but is typically discovered in ores. In its pure form, it appears as a silver metal with a grey coating.
Molybdic Acid Formula
Molybdic acid, also recognized as molybdenum hydroxide oxide, molybdic(VI) acid, or dihydrogentetraoxomolybdate, represents the hydrated variation of molybdenum trioxide and its associated compounds. It comes in two variations: monohydrate with the chemical formula MoO3·H2O and dihydrate with the formula MoO3·2H2O. This substance is diamagnetic and has a yellow color in its solid state.
Molybdic Acid Formula Name
The chemical formula for molybdic acid is MoO3·H2O, and its IUPAC name is "dihydrogentetraoxomolybdate."
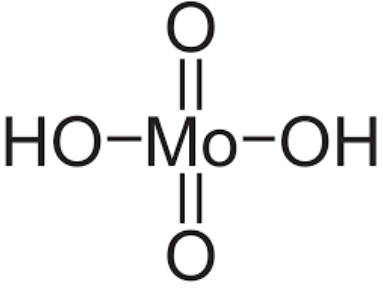
Molybdic Acid Formula Structure
Molybdic acid, in its hydrated form, corresponds to molybdenum trioxide and its associated compounds. Additionally, it goes by the names molybdenum hydroxide oxide and molybdic(VI) acid. The monohydrate has the chemical formula MoO3·H2O, while the dihydrate is MoO3·2H2O. Molybdic acid is classified as a molybdenum oxoacid and serves as the conjugate acid of hydrogenmolybdate. Its IUPAC name is dihydrogentetraoxomolybdate, and it has 2 hydrogen donors and 4 hydrogen acceptors in its structure.
Molybdic Acid Formula Preparation
To prepare molybdic acid, you can lower the pH of the molybdate ion to below 1, causing it to transform into molybdic acid. This can be achieved by adding 2 grams of ammonium molybdate tetrahydrate, (NH4)6Mo7O24·4H2O, to 28 mL of distilled water, followed by the removal of nitrogen.
Physical Properties of Molybdic Acid
- Molybdic acid is usually found in a solid or powder form.
- It has a pale yellow or white color.
- Relative density is 3.124.
- It is slightly soluble in water.
- Molybdic acid is highly soluble in alkaline solutions.
- The melting point of Molybdic acid is 300°C.
- It can dissolve in water at a rate of 70 grams per liter.
Chemical Properties of Molybdic Acid
- The molecular mass of molybdic acid is 161 atomic mass units (amu).
- Its molecular formula is MoO3·H2O.
- When pyrocatechol reacts with molybdic acid, it produces the MoO2 ion and 1,2-Cyclohexanedione.
Molybdic Acid Uses
Catalyst in Chemical Reactions: Molybdic acid plays a crucial role as a catalyst, enhancing the speed and efficiency of various chemical reactions. Its catalytic properties are valuable in numerous industrial processes.
Production of Froehde Reagent: Molybdic acid is an essential component in the formulation of Froehde reagent, a chemical solution used in various analytical and forensic tests, particularly for identifying and testing substances.
Precursor in Molybdenum Compounds: Molybdic acid serves as a precursor in the production of important compounds like molybdenum(IV) sulfide and ammonium molybdate tetrahydrate. These substances have various uses in a range of industries, such as metallurgy and agriculture.
Alkaloid Detection: When you mix molybdic acid with sulfuric acid, it helps detect certain chemicals called alkaloids.
Ammonium Molybdate Production: Molybdic acid is a key component in the manufacturing process of ammonium molybdate, a compound widely used in various applications, including corrosion inhibition and as a reagent in analytical chemistry.
Molybdic acid, a compound derived from molybdenum, is a versatile substance with a wide range of applications. It serves as a catalyst in chemical reactions, contributes to the formulation of important reagents like the Froehde reagent, and acts as a precursor in the production of various molybdenum compounds used in metallurgy and agriculture.
Additionally, molybdic acid finds value in the detection of alkaloids and plays a crucial role in the manufacturing of ammonium molybdate for applications in corrosion inhibition and analytical chemistry. Understanding the composition, structure, and properties of molybdic acid is essential for harnessing its diverse capabilities across different industries.
| Related Link | |
| Glucose Chemical Formula | Glutaric Acid Formula |
| Potassium Acetate Formula | Porpionic Acid Formula |
Molybdic Acid Formula FAQs










