
Glucose Chemical Formula a fundamental carbohydrate, has the chemical formula C 6 H 12 O 6 , denoting its molecular structure. It consists of six carbon (C) atoms, twelve hydrogen (H) atoms, and six oxygen (O) atoms arranged in a hexagonal ring. Glucose Chemical Formula is a monosaccharide, commonly called a simple sugar, and is the primary energy source for living organisms through cellular respiration. Its pivotal role in metabolism and energy production makes glucose a vital molecule in biological systems. Additionally, glucose molecules can bond through glycosidic linkages to form more complex carbohydrates, such as starch and cellulose, playing crucial roles in plant and human nutrition.
Glucose Chemical Formula (C 6 H 12 O 6 )
The glucose Chemical Formula is C 6 H 12 O 6 . Glucose Chemical Formula is simple sugar composed of six carbon atoms, twelve hydrogen atoms, and six oxygen atoms, and it is a carbohydrate that contributes to the body's metabolism.
The monosaccharide glucose, also known as dextrose or blood sugar, is produced by plants and most algae in photosynthesis. Sugars are a subgroup of carbohydrates, which are energy-giving compounds found in foods.
The process of oxidation of glucose chemical formula to carboxylic acid forms six carbon atoms. The oxidizing agent used in this is bromine water which is in the form of aldehyde group.The process of acetylation of glucose with acetic acid yields glucose pentaacetate. This confirms the presence of the -OH group.
Glucose Chemical Formula and Properties
Glucose is the most abundant organic compound. It is a monomer of many larger compounds, such as carbohydrates, starch, and cellulose. Here Below we will discuss about the glucose chemical formula and properties :
| Glucose Chemical Formula and Properties | |
| Glucose Chemical formula | C 6 H 12 O 6 |
| Molecular weight | 180.156 g/mol |
| Chemical names | dextrose, blood sugar, corn sugar and grape sugar |
| Molar mass | 180.156 g·mol-1 |
Glucose Chemical Formula Structure
A glucose chemical formula contains 6 carbon atoms and belongs to the aldehyde group. It can either be linear or cyclic. Glucose is the body's primary energy source.
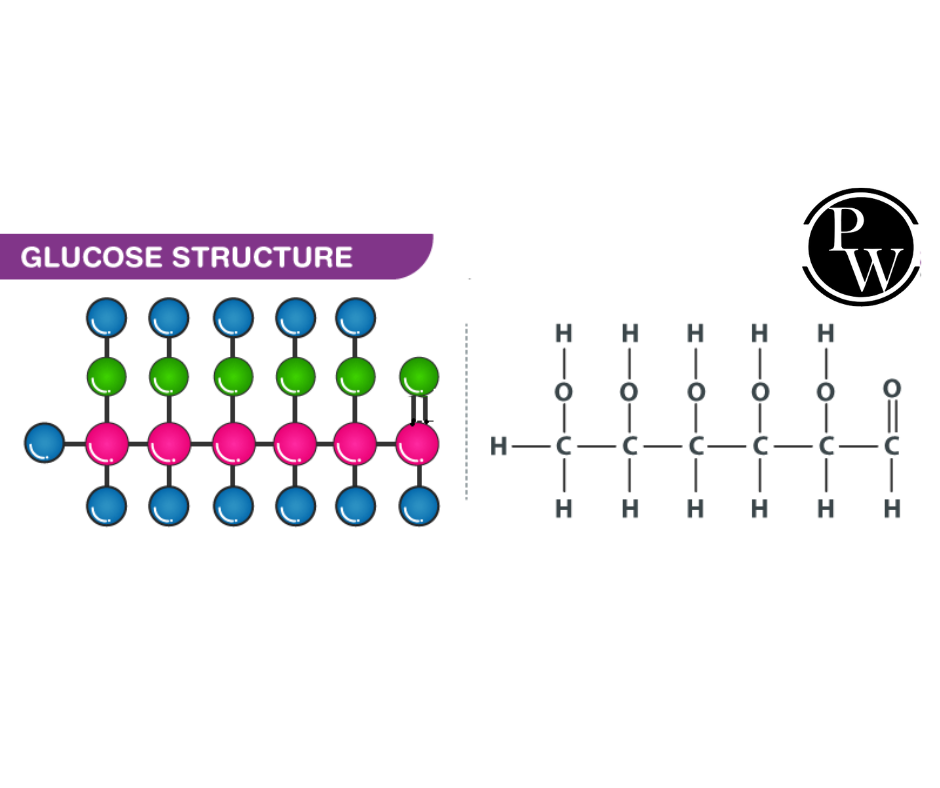
Open Chain: When glucose is in its open-chain form, its backbone is not open or branched, containing six carbon atoms.
Molecular Formula: Based on the analysis of elements in glucose and its molecular weight, glucose chemical formula C 6 H 12 O 6 .
Presence of a 6-carbon unbranched chain: Glucose contains an unbranched six-carbon chain, evidenced by its reduction with concentrated hydrogen iodide and red phosphorus.
Presence of 5 OH groups: When glucose reacts with acetic anhydride, pentadactyl derivatives form five hydroxyl groups. Since glucose is a stable molecule, two OH groups cannot bind to the same carbon, so there are five OH groups on various carbons. In a carbonyl group, an oxime is formed when glucose interacts with hydroxylamine.
Presence of terminal CHO function: The glucose molecule is mildly oxidized with bromine water to glucose acid, which is then reduced with HI to produce hexanoic acid.
The five OH groups can be placed on the remaining five carbons to form an open-chain glucose structure since glucose has a straight 6-carbon chain with a terminal CHO. Delivering hydrogen atoms to these carbon atoms meets their four valencies.
Also Read: Malic Acid Formula
Glucose Chemical Formula Equation
In order to breakdown glucose (C 6 H 12 O 6 ) (MW:180.156 g/mol), the following Glucose Chemical Formula Equation must be used:
Preparation From Sucrose or Cane Sugar
On boiling an aqueous solution of sucrose with dilute HCl or dilute H 2 SO 4 , glucose and fructose are formed in equal amounts.
C 12 H 22 O 11 (Sucrose) + H2O = C 6 H 12 O 6 (Glucose) + C 6 H 12 O 6 (Fructose)
Glucose Chemical Formula and Sucrose
The Chemical Formula for Sucrose is C12H22O11. A disaccharide, sucrose, is formed when glucose and fructose combine in a condensation reaction. Its equation is as follows:
C 6 H 12 O 6 + C 6 H 12 O 6 → C 12 H 22 O 11 + H 2 O
Glucose+ fructose → sucrose + water
Also Read: Tungstic Acid Formula
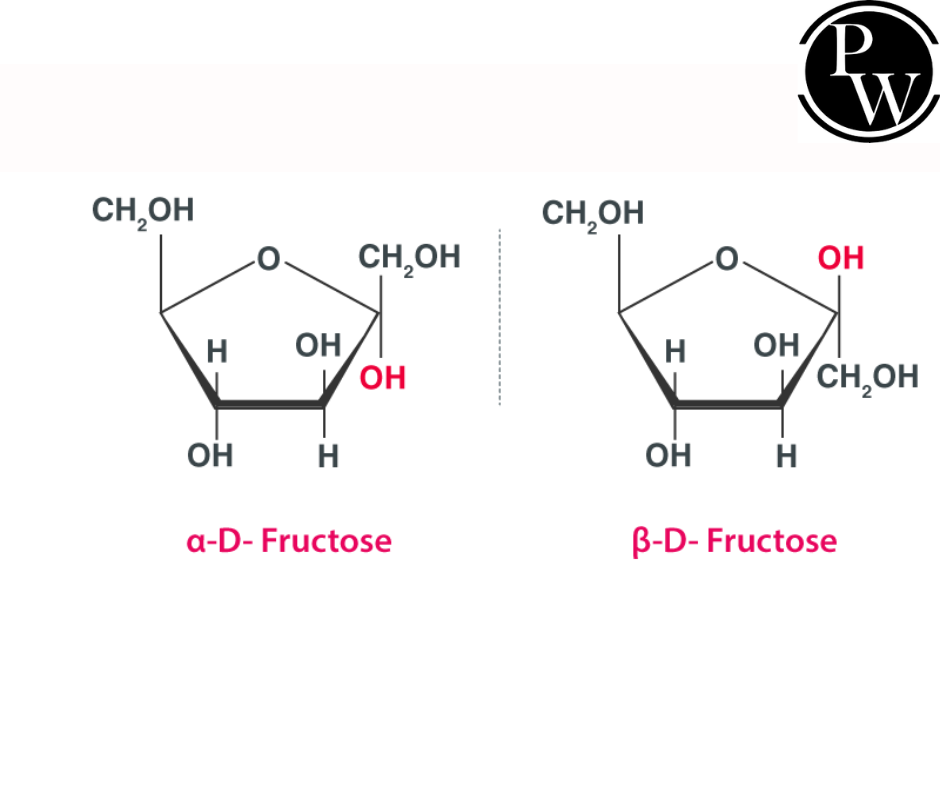
Glucose Chemical Formula and Fructose
Fructose is a monosaccharide with a chemical formula of C 6 H 12 O 6 and is also known as fruit sugar. It was discovered in 1847 by French chemist Augustin-Pierre Dubrunfaut.
As a result of the stability of the hemiketal and internal hydrogen bonds, crystalline fructose forms a cyclic six-membered structure called D-fructopyranose, which is found in honey, vine fruits, most root vegetables, berries, and flowers. Commercially, sugar beets, maize, and sugar cane are used to produce it.
Also Read: Acetone Formula
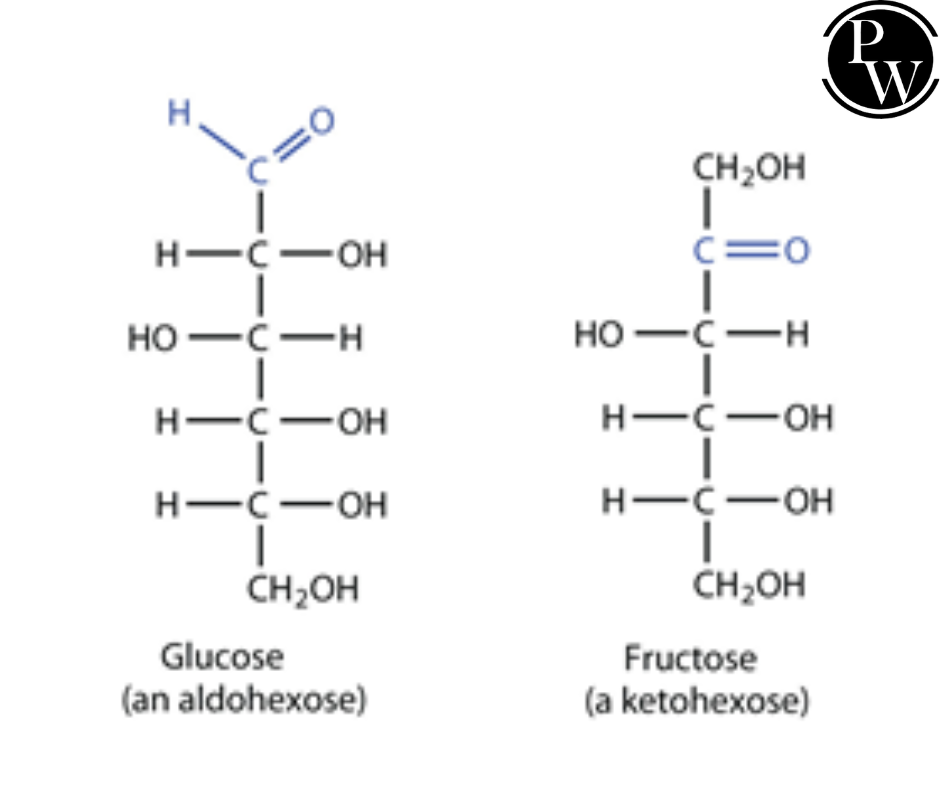
Glucose Chemical Formula Isomers
Glucose and Fructose are functional isomers of each other. The molecular formula of glucose and fructose is C6H12O6, but their chemical formulas have different functional groups. The functional group of glucose differs from the functional group of fructose. Glucose is an aldehyde, while fructose is a ketone. Glucose is an aldehyde, while fructose is a ketone.
| Related Links | |
| Acetaldehyde Formula | Amino Acid Formula |
| Acetylene Formula | Chloroform Formula |
Glucose Chemical Formula
Is C6H12O6 a glucose?
What is a C6H12O6 called?
What is the full formula of glucose?
Why is glucose c6?










