
The propionic acid formula is CH3CH2CO2. It is an oily liquid with a rotten smell; propionates or propanoates are salts and esters of propionic acid and anions. Propionic acid formula is a short-chain saturated fatty acid with a carboxy group attached to ethane; propionic acid is a short-chain saturated fatty acid.
Propionic Acid Formula Structure
Propionic acid, or propanoic acid, is a simple carboxylic acid with the chemical formula CH3CH2CO2. The propionic acid formula structure consists of three carbon atoms (C), six hydrogen atoms (H), and two oxygen atoms (O), arranged in a linear chain. The molecular formula can be written as CH3CH2COOH. This organic compound is part of the carboxylic acid family, characterized by a carboxyl group (COOH) at one end of the molecule.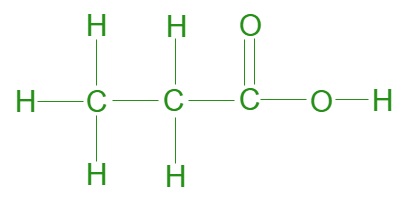
Also Read: Glycerol Formula
Propionic Acid Formula and Molecular Weight
The molecular weight of the propionic acid formula can be calculated by adding the atomic weights of its constituent elements. Carbon (C) has an atomic weight of approximately 12.01 g/mol, hydrogen (H) has an atomic weight of approximately 1.01 g/mol, and oxygen (O) has an atomic weight of approximately 16.00 g/mol. Using the formula C3H6O2, we can calculate the molecular weight as follows: Molecular weight of propionic acid (C3H6O2) = (3 × 12.01 g/mol) + (6 × 1.01 g/mol) + (2 × 16.00 g/mol) = 74.08 g/mol The molecular weight of propionic acid is approximately 74.08 grams per mole.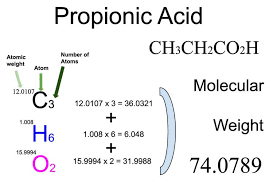
Propionic Acid Formula Compound
Propionic acid is a naturally occurring compound found in a variety of contexts. It is a carboxylic acid, which means it contains a functional carboxyl group (-COOH) responsible for its acidic properties. Propionic acid is commonly found in dairy products, especially cheeses, contributing to the tangy flavor. Additionally, it is produced in the human digestive system as a metabolic product of the fermentation of certain dietary carbohydrates.Also Read: Fumaric Acid
Properties of Propionic Acid formula
Propionic acid, with the chemical formula C3H6O2, possesses several important properties essential for its various industrial, chemical, and biological applications. Here are some of the critical properties of propionic acid:| Properties of Propionic Acid formula | |
| Propionic acid Formula | C3H6O2 or CH3CH2COOH |
| Molecular weight | 74.079 g/mol |
| Density of Propionic acid | 0.98797 g/cm3 |
| Chemical names | Propanoic Acid Ethanecarboxylic acid |
| Boiling point | 141.15 °C |
| Melting point | −20.5 °C |
| Crystal structure | Monoclinic |
Also Read: Aspirin Formula
Propionic Acid Formula and Uses
Food Preservation: Propionic acid and its salts (propionates) are used as preservatives in the food industry to prevent mold and bacteria growth in baked goods and other food products. They help extend the shelf life of bread, pastries, and other baked items. Animal Feed: Propionic acid is added to animal feed to inhibit the growth of mold and bacteria, which can spoil the feed and harm livestock. This ensures that the feed remains safe and nutritious for animals. Pharmaceuticals: Propionic acid and its derivatives produce various pharmaceuticals, including anti-inflammatory drugs and certain antibiotics. Plastics and Polymers: It serves as a precursor in producing several plastics and polymers, including cellulose acetate propionate (CAP) and polypropionate. Chemical Intermediates: Propionic acid is employed as a chemical intermediate in synthesizing other compounds, such as herbicides, perfumes, and flavors.Propionic Acid Formula Chemistry
The chemistry of propionic acid involves its reactions and properties as an organic acid. It is a weak acid that does not completely dissociate in water. In aqueous solutions, it partially ionizes into the propionate ion (C2H5COO-) and a hydrogen ion (H+). The equation represents this ionization: CH3CH2COOH ⇌ CH3CH2COO- + H+ Propionic acid reacts with bases to form propionate salts. It can also undergo esterification reactions to produce propionate esters utilized in various applications. Propionic acid is a vital organic compound with the chemical formula C3H6O2, known for its role in food preservation, animal feed, pharmaceuticals, and various chemical processes. Its simple structure and versatile chemistry make it a valuable ingredient in various industries. Understanding the propionic acid formula and its properties is essential for harnessing its potential in various applications.| Related Links | |
| Gluconic acid Structure Formula | Butane-1-ol Formula |
| Fumaric Acid Formula | Carbon Chemical Formula |
<span style=
How do you write propanoic acid?
Propanoic acid is typically written as CH3CH2COOH in its chemical formula. It is also known by its systematic IUPAC name, propanoic acid.
What is the common name of propionic acid?
The common name of propionic acid is simply "propionic acid." This name commonly refers to it in both scientific and industrial contexts.
Why is propionic acid used?
Propionic acid is used for various purposes, primarily as a preservative in the food industry to inhibit mold and bacterial growth, extending the shelf life of products like bread and cheese. It is also employed in animal feed to prevent spoilage and in the production of pharmaceuticals, plastics, and other chemical compounds.
Is propionic acid a chemical?
Yes, propionic acid is a chemical compound. It is an organic carboxylic acid with the chemical formula C3H6O2, used in numerous industrial and biological applications due to its versatile properties.
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









