
The formula for zinc iodide reflects the ratio of these atoms in the compound and is typically found in its ionic form. Zinc is a transition metal with the atomic number 30, and its most common oxidation state is +2. In the context of zinc iodide formula, zinc loses two electrons to become a Zn²⁺ cation. Iodine is a halogen element with the atomic number 53. It readily accepts an electron to form iodide ions (I⁻) when it reacts with metals like zinc.
Structure of Zinc Iodide Formula
The compound zinc iodide formula comprises two elements: zinc and iodine. Zinc is a brittle, silver-grey metal found in group 12 of the periodic table, with an atomic number of 30 and the symbol Zn. It is commonly used in various industries. On the other hand, Iodine is a lustrous dark grey solid non-metal in group 17, with an atomic number of 53 and symbol I. Among the elements in this group, iodine holds the highest degree of positivity and the lowest reactivity.
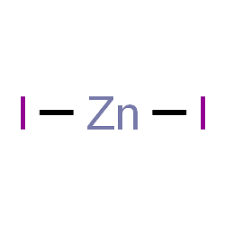
Zinc Iodide Formula for Ionic Compound
The formula for zinc iodide, when it exists in its ionic compound form, can be expressed as ZnI2. This representation reflects the stoichiometry of the compound, indicating that it consists of one zinc cation (Zn^2+) and two iodide anions (I^-).
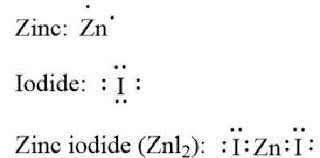
Zinc (Zn): Zinc is a transition metal with a 2+ charge when it forms ions. In the case of zinc iodide, it donates two electrons to become a Zn^2+ cation.
Iodine (I): Iodine is a halogen element that readily accepts an electron to form iodide ions (I^-) when reacting with metals like zinc.
When these ions combine, they form zinc iodide in a 1:2 ratio, with two iodide ions balancing the charge of the single zinc ion.
Zinc Iodide Formula (Ionic)
The zinc iodide formula in its ionic form can be represented as ZnI2. This compound is held together by the electrostatic attraction between the positively charged zinc ions and the negatively charged iodide ions. The balanced charge between the ions ensures the stability of the ionic compound.
The ionic nature of zinc iodide makes it soluble in water, and it can conduct electricity when dissolved or in molten form due to the presence of mobile ions.
Zinc Iodide Formulation
Zinc iodide is commonly formulated as a white, crystalline solid, although it may appear yellowish due to impurities. It has various applications in chemistry, medicine, and electronics industries. Some common uses include as a reagent in chemical synthesis, a component in photographic chemicals, and in radiographic imaging.
Zinc Iodide Properties
Here are some of the properties of Zinc Iodide :| Properties of Zinc Iodide | |
| Name | Zinc Iodide |
| Appearance | White powder |
| Chemical Formula | ZnI 2 |
| Melting Point | 446 °C |
| Boiling Point | 1,150 °C |
| Density | 4.74 g/cm³ |
| Molar Mass | 319.22 g/mol |
Zinc Iodide Uses
Zinc iodide has several important uses in various fields due to its unique properties. Here are some of the most common applications of zinc iodide:- Organic Reactions: Zinc iodide is used as a Lewis acid catalyst in various organic reactions. It can promote reactions like the Friedel-Crafts alkylation and acylation, leading to the synthesis of a wide range of organic compounds.
- Photographic Chemicals: In the past, zinc iodide was used in photographic emulsions as a sensitizer for silver halide crystals. It helped enhance the sensitivity of the emulsion to light.
- X-ray Screens: Zinc iodide scintillators are employed in radiographic imaging, such as X-ray screens. These screens convert X-ray photons into visible light, making it possible to capture detailed X-ray images.
- Phosphors: Zinc iodide can be used as a phosphor material in cathode ray tubes (CRTs) and other display technologies. It emits visible light when exposed to high-energy electrons or other forms of radiation, making it useful in various types of electronic displays
- Analytical Chemistry: Zinc iodide can be used in qualitative chemical analysis to detect the presence of certain functional groups in organic compounds. It can form specific complexes with various functional groups, aiding in the identification of unknown substances.
- Radiopharmaceuticals: Some radiopharmaceuticals used in nuclear medicine may contain zinc iodide compounds. These compounds can help localize and visualize specific tissues or organs in diagnostic imaging.
- Colorants: Zinc iodide can be used as a source of iodine in the preparation of certain dyes and pigments. It contributes to the coloration of these materials.
| Related Links | |
| Barium Acetate Formula | Barium Nitrate Formula |
| Barium Chloride Formula | Barium Phosphate Formula |
Zinc Iodide Formula FAQs
What is zinc iodide formula?
How is zinc iodide formed?
What is the equation for zinc iodide formation from its ions?
What is ZnI2 called?










