
Barium phosphate formula, an inorganic salt, has the chemical formula Ba3(PO4)2. It is a colourless solid with a faint odour reminiscent of acetic acid. Using X-ray crystallography, researchers determined that the compound consists of barium ions bound to polyphosphate anions. These polyphosphate anions are salts or esters of tetrahedral PO4 (phosphate) units linked by shared oxygen atoms. Barium phosphate exists in multiple hydrated forms, including Barium Phosphate Dihydrate.
The barium phosphate formula is Ba3(PO4)2, which is a compound composed of barium cations (Ba2+) and phosphate anions (PO43-). This chemical compound plays a role in various applications, including manufacturing ceramics, phosphors for electronic displays, and as a reagent in some chemical reactions.
Barium Phosphate Formula
Barium phosphate, also known as tribarium diphosphate, has the formula Ba3(PO4)2. This compound consists of 3 barium cations and 2 phosphate anions. The cations are provided by the barium atom while PO43 supplies the anions-. In line with IUPAC nomenclature, it is called barium(2+); diphosphate. The structural formula is used to understand the stereochemistry of this salt.
Also Read: Aldehyde & Ketone
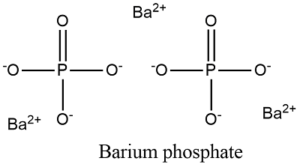
Barium Phosphate Formula by Criss Cross Method
The formula for barium phosphate can be determined using the criss-cross method, a straightforward approach for balancing the charges of ions in a chemical compound. Barium has a 2+ charge (Ba2+), and phosphate has a 3- charge (PO43-). To balance these charges, you can cross the numerical charges of the ions, leading to the formula Ba3(PO4)2, where the subscript numbers indicate the number of each ion needed to balance the charges.
Barium Phosphate Formula Weight
The formula weight (also known as molar mass) of barium phosphate, Ba3(PO4)2, is calculated by summing the atomic masses of all the atoms in the compound. For Ba3(PO4)2, this includes the atomic masses of three barium atoms (Ba) and two phosphate groups (PO4). The formula weight is crucial for various chemical calculations.
Barium Phosphate Formula Mass
The formula mass of Ba3(PO4)2, which is the same as its formula weight, can be calculated by adding up the atomic masses of its constituent atoms. Barium has an atomic mass of approximately 137.33 g/mol, and each phosphate group (PO4) has an atomic mass of around 94.97 g/mol. You can calculate the formula mass by summing these values for the compound's three barium atoms and two phosphate groups.
Barium Phosphate Formula Solid
Barium phosphate formula is typically encountered as a solid material. Depending on its application, it may appear as a white, crystalline powder or in various other forms. Handling this solid compound carefully is essential, as it can be hazardous when not used appropriately.
Barium Phosphate Formula Units
The formula Ba3(PO4)2 indicates that in one unit of barium phosphate, there are three barium ions (Ba2+) and two phosphate ions (PO43-). These units repeat in the crystal lattice structure of the solid compound, creating a three-dimensional network of positively charged barium cations and negatively charged phosphate anions.
Also Read: HydrocarbonBarium Phosphide Formula
Barium phosphide is a different compound than barium phosphate. Barium phosphide has the chemical formula Ba3P2 and consists of three barium cations (Ba2+) and two phosphide ions (P3-). It is a binary ionic compound, contrasting with the more complex barium phosphate.
Barium Phosphide Formula Compound
Barium phosphide, Ba3P2, is a binary compound of barium and phosphide ions. It is used in some chemical reactions and has specific applications in semiconductors and electronics.
Barium Dihydrogen Phosphate Formula
Barium dihydrogen phosphate, or barium bis(dihydrogen phosphate), has the chemical formula Ba(H2PO4)2. It contains barium cations (Ba2+) and dihydrogen phosphate anions (H2PO4-). This compound is used in various industries, including agriculture and as a source of phosphorus in fertilizers.
Barium Phosphate Properties
Below are some of the physical properties of barium phosphate, such as its color, state, melting point, boiling point, and appearance. Barium phosphate has a melting point of 1560 oC and a density of 3.63 g/mL. Barium phosphate is a colourless compound which is generally in powder form. It is not soluble in water. It can be dissolved in some acids, such as acetic acid.
Use and Safety Measure of Barium Phosphate
The Barium Phosphate Formula, Ba3(PO4)2, has various industrial uses ranging from producing sodium and barium polyphosphate for specialized glasses to functioning as a proton-conducting material in pulsed lasers. Recent research has also shown that it can coat magnesium alloys with phosphate coatings during chemical conversion, resulting in the formation of MgF2. This study further indicates that Barium Phosphate exhibits improved potential increases and decreases in corrosion current density compared to calcium phosphate. It is important to note that this substance should not be consumed or inhaled as it can be harmful and may cause irritation to the eyes, mouth, mucous membranes, and skin. In case of ingestion, seek immediate medical attention.
| Related Links | |
| Electrophiles | Acetone formula |
| Nucleophile formula | Actetamide formula |
Barium Phosphate Formula FAQs
How do you write barium phosphate?
What is the formula name for barium phosphate?
What is the valency of barium phosphate?
What is the symbol for barium phosphate?










