
Barium acetate formula is a chemical compound with the formula Ba(CH3COO)2. This article will delve into various aspects of barium acetate, including its formula, ionic nature, name, molar mass, hydrate form, molecular formula, polyatomic structure, solid-state formula, and its role in reactions with potassium phosphate.
Barium Acetate Formula of Ionic Compound
Barium acetate is an ionic compound, forming through the combination of cations (positively charged ions) and anions (negatively charged ions). In this case, the barium ion (Ba²⁺) combines with the acetate ion (CH3COO⁻) to create a stable ionic compound. This ionic nature is essential for its solubility and reactivity in various chemical reactions.Also Read: Urethane formula
Barium Acetate Formula Name
The formula name "barium acetate" accurately reflects the chemical composition of this compound. "Barium" represents the metal cation, Ba²⁺, and "acetate" signifies the acetate anion, CH3COO⁻. Barium acetate is commonly used in laboratory settings and industrial applications.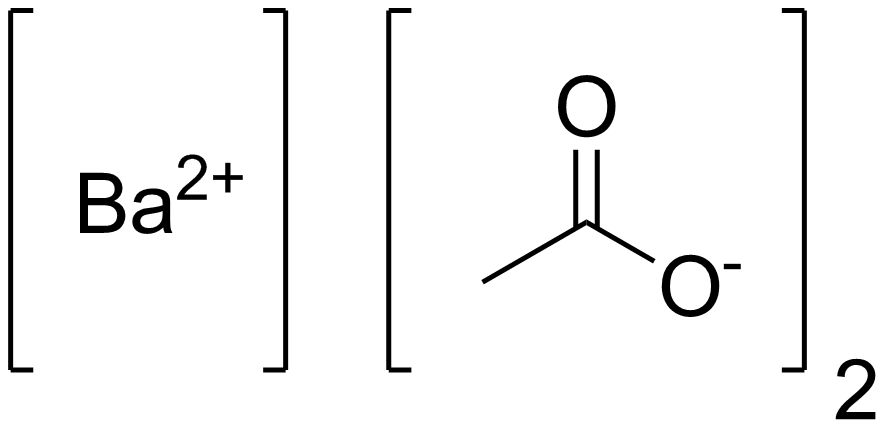
Barium Acetate Molar Mass
To calculate the molar mass of barium acetate (Ba(CH3COO)2), we must consider the atomic masses of its constituent elements. Barium (Ba) has a molar mass of approximately 137.33 g/mol, while the molar mass of acetate (CH3COO⁻) is approximately 59.04 g/mol. By adding these values together, we can determine the molar mass of barium acetate, which is approximately 254.41 g/mol.Barium Acetate Dihydrate Formula
Barium acetate can exist in a dihydrate form, which means it contains water molecules in its structure. The dihydrate form of barium acetate has the chemical formula Ba(CH3COO)2·2H2O. The presence of water molecules makes it suitable for applications where controlled release of acetate ions is necessary.Also Read: Cinnamic Acid Formula
Barium Acetate Molecular Formula
The molecular formula of barium acetate is Ba(CH3COO)2, which provides information about the actual number of atoms of each element in a single compound molecule. This formula is essential for understanding its stoichiometry and structural characteristics.Barium (II) Acetate Formula
The Roman numeral "II" in the name "barium (II) acetate" indicates the charge on the barium ion, Ba²⁺, in the compound. The acetate ion, CH3COO⁻, remains the same. Therefore, the formula for barium (II) acetate is Ba(CH3COO)2, and it distinguishes it from other possible barium acetate compounds with different oxidation states of barium.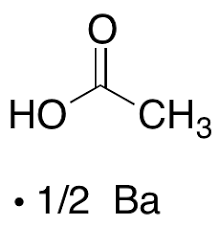
Barium Acetate Polyatomic Formula
The formula for barium acetate features a polyatomic ion, acetate (CH3COO⁻). The acetate ion is composed of carbon (C), hydrogen (H), and oxygen (O) atoms. It acts as a single, negatively charged unit within the compound, facilitating the formation of the ionic compound with the barium cation.Solid Barium Acetate Formula
The formula for solid barium acetate remains the same as the compound in any other state. In the solid state, barium acetate is a white crystalline powder, maintaining the chemical formula Ba(CH3COO)2.Also Read: Thiourea formula
Barium Acetate and Potassium Phosphate Formula
When barium acetate and potassium phosphate react, they form barium phosphate and potassium acetate. The balanced chemical equation for this reaction is: Ba(CH3COO)2 + K3PO4 → Ba3(PO4)2 + 3KCH3COO This reaction illustrates the exchange of ions between the two compounds, leading to the formation of barium phosphate and potassium acetate.Barium Acetate Properties
It has the appearance of a white crystal or powder. It is very soluble in water and has a solubility of 59 g/100ml at a temperature of 20 °C. The density of barium acetate is 2.47 grams per square centimeter. When heated, this salt decomposes into barium carbonate.Barium Acetate Uses
Experts utilize barium acetate as a mordant for printing textile fabrics and as an excellent paint drying agent. It also serves as a lubricating oil additive in the field of chemistry and is involved in the production of other acetates. Furthermore, this versatile salt acts as a catalyst in organic synthesis according to experts. Despite its many applications, it's important to note that there are potential health hazards associated with Barium Acetate Formula. If swallowed or inhaled, it can be dangerous and irritate the eyes, skin, and respiratory tract. This salt can also target various organs such as the lungs, heart, muscles, gastrointestinal system, and nerves. In extreme cases, ingestion of barium acetate can be fatal due to its muscle poison properties causing muscle stimulation and eventual paralysis.| Related Links | |
| Acetone formula | Ascorbic Acid formula |
| Actetamide formula | Butane Formula |
Barium Acetate Formula FAQs
What is the formula for acetate?
The formula for acetate is CH3COO⁻, representing a polyatomic ion known as the acetate ion.
What type of compound is barium acetate?
Barium acetate is an ionic compound. It is composed of positively charged barium ions (Ba²⁺) and negatively charged acetate ions (CH3COO⁻).
What is the formula for barium acetate in polyatomic ions?
The formula for barium acetate is Ba(CH3COO)2, where the polyatomic ion "acetate" (CH3COO⁻) remains intact.
What is the name of the compound Ba(C2H3O2)2?
The compound Ba(C2H3O2)2 is called "barium acetate."
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









