
The Barium Nitrate Formula is Ba(NO 3 ) 2 . It is colourless, water-soluble, and toxic. It burns with a green flame and is commonly used in pyrotechnics. In nature, it occurs as a mineral known as nitrobarite. This article will provide an overview of the Barium Nitrate formula, its properties and chemical structure, and its uses.
Barium Nitrate Formula
The chemical formula for barium nitrate is Ba(NO 3 ) 2 . This formula reveals the specific arrangement of atoms in a single molecule of barium nitrate, consisting of one barium ion (Ba²⁺) and two nitrate ions (NO3⁻).
Similarly, Barium Nitrate has a molar mass of 261.34 g mol-1. The chemical formula is Ba(NO 3 ) 2 . Looking at the structure, we can see that it is composed of one Barium cation Ba2+ and two nitrate anions NO3–. Moreover, the crystal structure is cubic with one barium cation surrounded by four nitrate anions. You can write the chemical structure of Barium Nitrate by following the image given below, in the manner common for organic molecules.
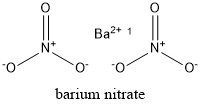
Barium Nitrate Formula Ions
The ions within the barium nitrate formula are as follows: - Barium ion (Ba²⁺): This is a cation, or positively charged ion, carrying a double positive charge. - Nitrate ion (NO3⁻): Nitrate is an anion, a negatively charged ion, with a single negative charge. It consists of one nitrogen (N) atom bonded to three oxygen (O) atoms.
Barium Nitrate Formula by Crisscross Method
The crisscross method is a practical technique for determining the chemical formula of an ionic compound. In the case of barium nitrate (Ba(NO 3 ) 2 ), this method involves "crisscrossing" the numerical charges of the ions. Barium has a charge of +2 (Ba²⁺), while nitrate carries a charge of -1 (NO3⁻). We obtain the correct formula Ba(NO 3 ) 2 by swapping these numerical charges.
Barium Nitrate Formula and Charge
The chemical formula for barium nitrate, Ba(NO 3 ) 2 , accurately represents both the chemical constituents and their charges. The barium ion (Ba²⁺) has a charge of +2, and each nitrate ion (NO3⁻) has a charge of -1. The combination of one Ba²⁺ ion with two NO3⁻ ions results in a neutral compound.
Barium Nitrate Formula Mass
To determine the formula mass of barium nitrate (Ba(NO 3 ) 2 ), the atomic masses of its individual elements must be considered. Barium (Ba) has an atomic mass of around 137.33 g/mol, nitrogen (N) has an atomic mass of approximately 14.01 g/mol, and oxygen (O) has an atomic mass of roughly 16.00 g/mol. By summing these values and accounting for the two nitrate ions, we find that the formula mass is 261.34 g/mol.
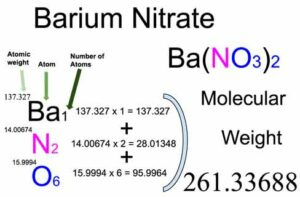
The formula for Barium Nitrate
The chemical formula for barium nitrate is specifically represented as Ba(NO 3 ) 2 , and this is crucial for accurately denoting its chemical structure.
Barium Nitrate Formula Unit
The formula unit for barium nitrate, Ba(NO 3 ) 2 , represents the smallest, electrically neutral component of the compound. It consists of one barium ion (Ba²⁺) and two nitrate ions (NO3⁻).
Barium Nitrate Formula Atoms
The formula Ba(NO 3 ) 2 encompasses eight atoms within a single molecule. These include one barium atom (Ba), one nitrogen atom (N), and six oxygen atoms (O) from the two nitrate ions.
Barium Nitrate Formula Sodium Sulfate
Barium nitrate, with the formula Ba(NO 3 ) 2 , should not be confused with sodium sulfate. Sodium sulfate is a distinct chemical compound represented by the formula (Na 2 SO 4 ), and it has different chemical properties and applications.
Barium Nitrate Formula Química
"Barium Nitrate Formula Química" refers to the chemical formula of barium nitrate in the Spanish language. In this context, "fórmula química" translates to a chemical formula, and barium nitrate is accurately expressed as Ba(NO 3 ) 2 .
Physical Properties of Barium Nitrate Formula
The colour of Barium Nitrate is shiny white and it is found in crystalline solid form. The density of it is 3.24 g mL -1 . Further, the melting point of this salt is 592 °C. Thus, if kept in above the given temperature, it will decompose. Moreover, it is said to be highly soluble in hot water and poorly soluble in cold water. Similarly, Barium Nitrate is not soluble in ethanol. The colour of the flame when it burns is green.
Chemical Properties of Barium Nitrate Formula
It is extremely strong oxidizing agent, which makes it very easy to oxide some metals and oxides, which react very aggressively with them. Due to its high Nitrate content, Barium Nitrate is often used for military purposes. For instance, people use it to make grenades and other types of explosives. In the same vein, when it is exposed to high temperatures, it produces Barium Oxide and NO2.
Uses of Barium Nitrate
In general, it is used in pyrotechnics, where the compound produces the green flames that we see. Additionally, it can also be used in the manufacture of some materials containing Barium Oxide. Additionally, it is used to create explosives such as Baratol, Thermate-TH3 and many others. It is also used in green signal lights, detonators, propellants, paints, rodenticides, and others.
| Related Links | |
| Calcium Bromide Formula | Carbon Monoxide Formula |
| Calcium Iodide Formula | Chloroacetic Acid Formula |
Barium Nitrate Formula FAQs
What is the formula for barium nitrate?
What is another name for barium nitrate?
How do you write Ba(NO3)2?
What does barium nitrate do?










