
Electromagnetic Wave Theory : Welcome, curious minds, to the captivating world of wave theory! In this article, we'll embark on an exciting journey to unravel the mysteries of waves and explore their fundamental characteristics. From understanding wavelength and frequency to delving into wave number and amplitude, we'll break down these concepts into simple terms that anyone can grasp. So, let's dive in and discover the fascinating world of waves.
In the quantum world, particles such as electrons exhibit wave-like behaviour, as described by the principles of wave-particle duality. This wave-particle duality implies that particles, including electrons, can exhibit both particle-like and wave-like properties. This wave-like behaviour is governed by wave equations, such as the Schrödinger equation, which describe the behaviour of particles in terms of wave functions. Now, let's explore the fundamental characteristics of waves and their implications in atomic structure:
- It is the energy transmitted from one body to another in the form of waves and these waves travel in the space with the same speed as light (3 × 10 8 m/s) and these waves are known as Electromagnetic waves or radiant energy.
- The radiant energy do not need any medium for propagation.
Example of Electromagnetic waves
Radio waves, micro waves, infra red rays, visible rays, ultraviolet rays, x-rays, gama rays and cosmic rays.
- A wave is characterized by following six characteristics. The upper most point of the wave is called crest and the lower most point is called trough . Some of the terms employed in dealing with the waves are described below:
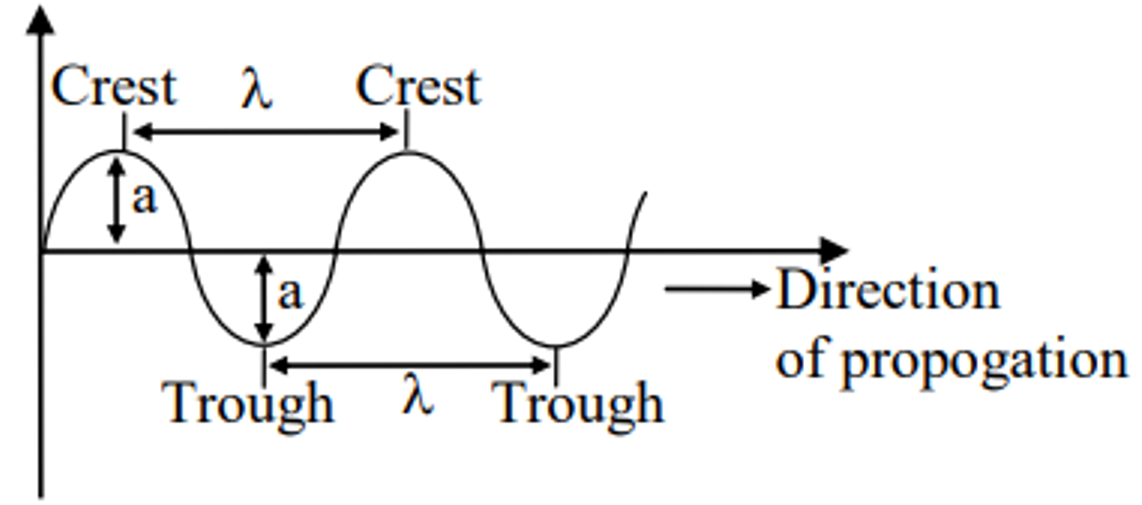
Wavelength (λ):-
Wavelength represents the spatial extent of the wave function associated with an electron in an atom. It signifies the distance between adjacent peaks (or troughs) of the electron's wave function. The wavelength of an electron is inversely proportional to its momentum, as described by de Broglie's equation:
λ = h / p
Where,
λ is the wavelength
h is Planck's constant
p is the momentum of the electron.
It is measured in terms of a Å (Angstrom), pm (Pico-meter), nm (nano-meter), cm (centi-meter), m (meter)
1Å = 10 –10 m, 1 pm = 10 –12 m, 1 nm = 10 –9 m, 1 cm = 10 –2 m
Frequency (ν):-
Frequency relates to the energy of the electron and is directly proportional to its kinetic energy. In atomic structure, frequency signifies the number of oscillations or cycles of the electron's wave function per unit time. Higher energy electrons exhibit higher frequencies, corresponding to shorter wavelengths, as described by the relationship:
E = hν ,
Where, E is the energy of the electron.
It is measured in terms of Hertz (Hz), sec –1 , or cycle per second (cps)
1 Hertz = 1 sec –1 = 1 cps
Time Period (T):-
The time period represents the time taken for one complete cycle of the electron's wave function. In the context of atomic structure, the time period provides insights into the dynamic behavior of electrons within atoms. It is the reciprocal of frequency, described by the equation: T = 1/ ν .
In short, Time Period is the Time taken by a wave to pass through one point .
Wave number
: It is the reciprocal of the wave length that is number of waves present in 1 cm
1 m = 100 cm
(1 cm –1 = 100 m –1 )
It is measured in terms of cm –1 , m –1 etc. Amplitude (A) : The amplitude of a wave is defined as the height of crust or depth of trough.Solved Examples of Electromagnetic Wave Theory
Q.1:
Calculate
in cm
–1
and
v
of yellow radiations have wavelength of 5800 Å.
Sol.
3 × 10
10
cm s
–1
× 1.7 × 10
4
cm
–1
= 3 × 1.7 × 10
14
= 5.1 × 10
14
s
–1
Q.2 : A Particular radio station broadcast at a frequency of 1120 Kilo Hertz another radio station broadcast at a frequency of 98.7 megahertz. What are the wave length of radiations from each station?
Sol :
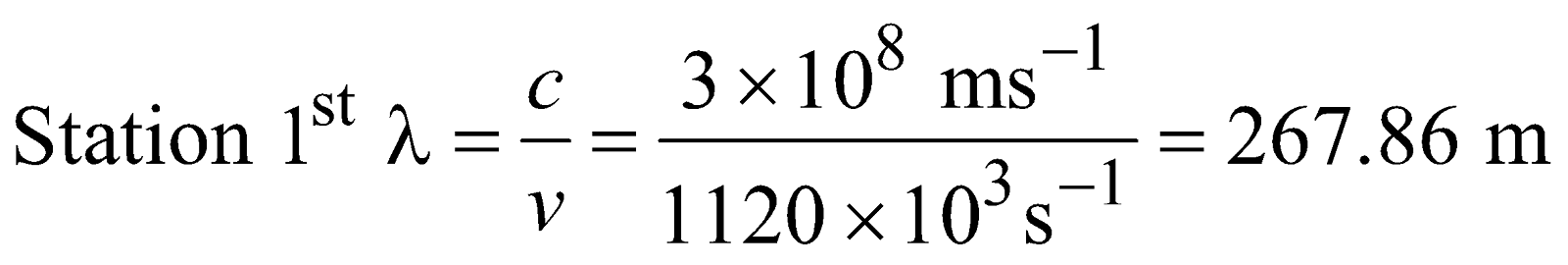
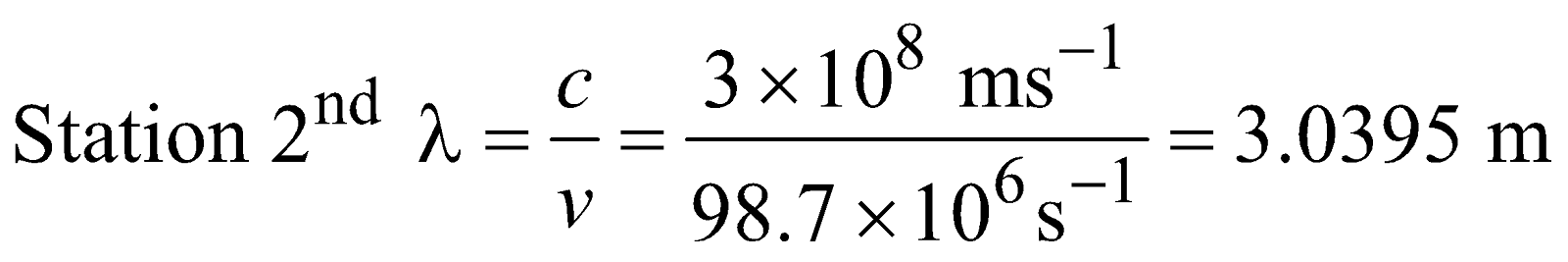
Q.3 : What will be the frequency of photon of wavelength 2225 Å traveling in vacuum?
Sol : Velocity of light in vacuum = 3 × 10 8 m sec –1
Wavelength = 2225 × 10 –10 meter
![]()
= 1.349 × 10 15 sec –1
Electromagnetic Wave Theory FAQs
Q.1 : What is wavelength, and how is it important in chemistry?
Q.2 : What is frequency, and why does it matter in chemistry?
Q.3 : How do wavelength and frequency relate to each other?
Q.4 : What is amplitude, and why is it important in wave theory?
Q.5 : What is the significance of wave number in chemistry?
Q.6 : How do time period and frequency relate to each other in wave theory?










