
CBSE Class 12 Chemistry Answer Key 2024: The Central Board of Secondary Education (CBSE) conducted the CBSE Class 12 Chemistry Exam 2024 on February 27, 2024, at various exam centers. The exam took place from 10:30 am to 01:30 pm, with an additional 15 minutes provided for students to review the question paper.
Now that the Class 12 Chemistry exam has been conducted, all students who appeared for the CBSE Chemistry Board Examination 2024 can access the complete CBSE Class 12 Chemistry Answer Key 2024 PDF with Question Paper for all sets 1, 2, 3, and 4 via the direct link provided in the article below.CBSE Class 12 Chemistry Answer Key 2024
The CBSE Class 12th Chemistry Board Examination 2024 was scheduled to be conducted on February 27, 2024. Students were allocated a time duration of 3 hours to complete the exam, with an additional 15 minutes provided for reading the question paper. Chemistry is very challenging subject for students due to its extensive scope and combination of descriptive and mathematical aspects.CBSE Class 12 Physics Answer key 2024
After the CBSE Class 12 Chemistry Exam 2024, students can find the complete Class 12 Chemistry answer key 2024 here. However, it is important to note that while the answer key can provide assistance, it may not always be entirely accurate. Therefore, cross-checking with official sources is highly recommended for accurate results.CBSE Class 12 Biology Answer Key 2024
Here is the overview of CBSE Class 12 Chemistry Answer Key 2024| Details | Information |
|---|---|
| Exam Conducting Body | Central Board of Secondary Education |
| Name of the Examination | CBSE Class 12th Board Examination 2024 |
| Category | Answer Key |
| Status | Released |
| CBSE Class 12 Chemistry Exam Date 2024 | February 27, 2024 |
| Class 12 Chemistry Answer Key 2024 Release Date | February 27, 2024 |
| Chemistry 12th Total Marks | 70 marks (Theory: 70 marks, Practical: 30 marks) |
| Negative Marking | No negative marking |
| Mode of Answer Key Availability | Online Mode |
| Official Website | CBSE Official Website |
Class 12 Chemistry CBSE Answer Key 2024 All Sets
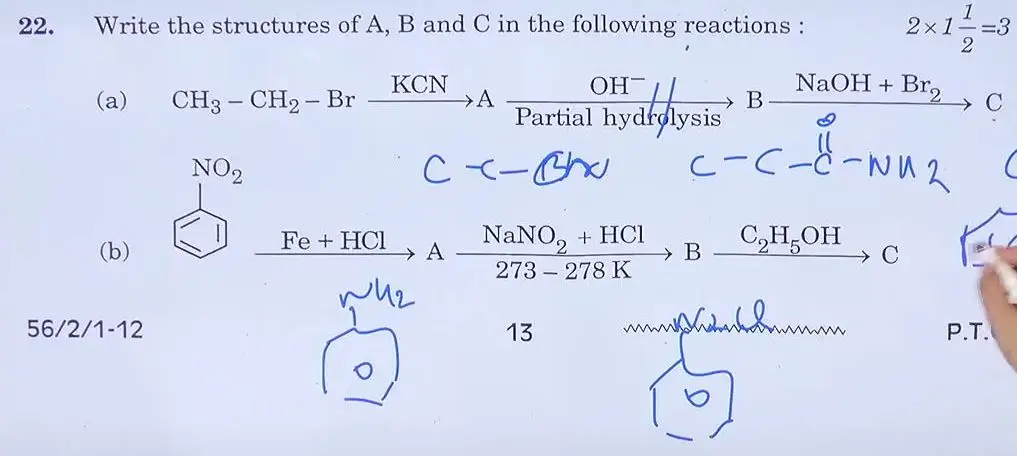
We here provided all the SET 1, 2, 3, 4 CBSE Class 12th Chemistry Answer Key 2024 for students to help them analyse the exam paper.CBSE Class 12 Chemistry Answer Key 2024 SET 1 56/2/1
SECTION A
1. When MnO2 is fused with KOH in air, it gives (A) KMnO4 (B) K2MnO4 (C) MngO7 (D) Mn2OAnswer: (B) K2MnO4
2. Ligand EDTA is an example of a: (A) Monodentate ligand (B) Didentate ligand (C) Tridentate ligand (D) Polydentate ligandAnswer:(D) Polydentate ligand
3. Which of the following ligand forms chelate complex? (A) C2O2-4 (B) CI (C) NO (D) NH3Answer: (A) C2O2-4

Answer: (C)
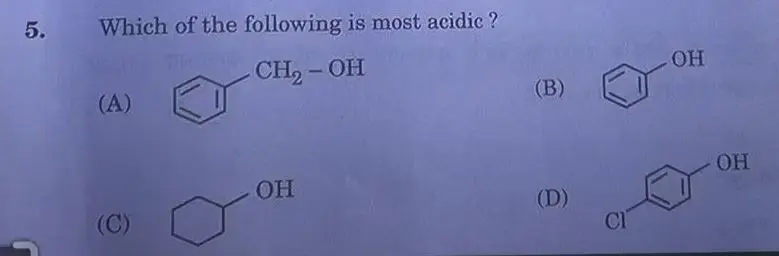
Answer: (B)
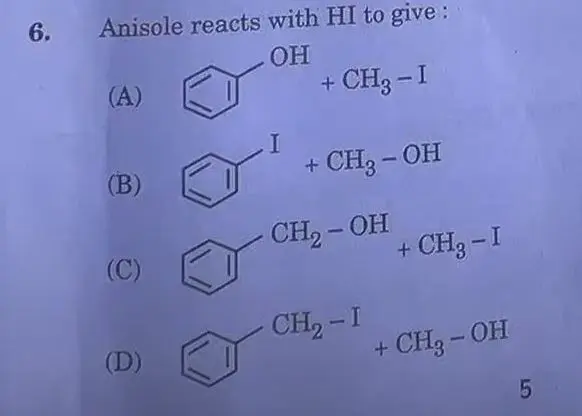
Answer: (A)
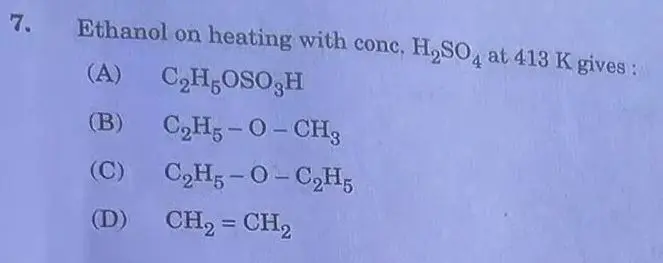
Answer: (D)
8. An Azetrophic Solution of two liquids has boiling point lower than either of them when it (A) is saturated (B) shows positive deviation from Raoult’s law (C) shows negative deviation from Raoult’s law (D) shows no deviation from Raoult’s lawAnswer: (B) shows positive deviation from Raoult’s law
9. The relative lowering of vapour pressure of an aqueous solution containing non-volatile solute is 0.0225. The mole fraction of the non-volatile solute is: (A) 0.80 (B) 0.725 (C) 0:15 (D) 0.0225Answer: (D) 0.0225
10. During electrolysis of aqueous solution of NaCl : (A) H2 (g) is liberated at cathode (B) Na is formed at cathode (C) O2 (g) is liberated at anode (D) Cl2 (g) is liberated at cathodeAnswer: (A) H2 (g) is liberated at cathode
11. The addition of catalyst during a chemical renètion alters which of the following quantities of the reaction? (A) Enthalpy (B) Activation energy (C) Entropy (D) Internal energyAnswer: (B) Activation Energy
12. For the elementary reaction PQ, the rate of disappearance of ‘P’ increases by a factor of 8 upon doubling the concentration of ‘P’. The order of the reaction with respect to ‘P’ is: (A) 3 (B) 4 (C) 2 (D) 1Answer: (A) 3
13. Assertion (A): Aliphatic primary amines can be prepared by Gabriel phthalimide synthesis. Reason (R): Alkyl halides undergo nucleophilie substitution with anion formed by phthalimide.Answer: (A) Both true
14. Assertion (A): Uracil base is present in DNA. Reason (R): DNA undergoes self-replication.Answer: (D) Assertion is False, But Reason is True
15. Assertion (A): Diazonium salts of aromatic amines are more stable than those of aliphatic amines. Reason (R): Diazonium salts of aliphatic amines show resonance.Answer: (C)
16. Assertion (A): p-nitroaniline is a weaker base than p-toluidine. Reason (R): The electron withdrawing effect of NO2 group in p-nitroaniline makes it a weaker base. Answer: (A) Both true 21. Define the following terms:
(a) Denaturation of protein
21. Define the following terms:
(a) Denaturation of protein
Answer: Denaturation of Protein happens when a protein's natural structure changes without its peptide bonds breaking. This usually occurs when the protein's secondary, tertiary, or quaternary structures get disturbed, making it lose its biological function. Denaturation can happen due to factors like high heat, extreme pH levels, certain chemicals, or rough handling. When denaturation occurs, the protein unfolds and loses its usual three-dimensional shape, which is necessary for its proper function.
(b) Invert SugarAnswer: Invert sugar is a mixture of two simple sugars: glucose and fructose. It is formed by the hydrolysis (breakdown) of sucrose, which is a disaccharide composed of one molecule of glucose and one molecule of fructose linked together.
CBSE Class 12 Chemistry Answer Key 2024 SET 2 56/4/2
SECTION A
1. The molar ionic conductivities of Mg2+ and SO2 are 106.0 S cm² mol-¹ and 160.0 S cm² mol-¹ respectively. The value of limiting molar conductivity of MgSO4 will be : (A) 266 S cm² mol-1 (C) 288 S cm² mol-1 (B) 622 S cm² mol-1 (D) 822 S cm² mol-1Answer: (A) 266 S cm² mol-1
2. From the elements of 3d series given below, which element shows the maximum number of oxidation states? (A) Scandium (C) Chromium (B) Manganese (D) TitaniumAnswer: (B) Manganese

Answer: (A)
4. Which of the following acids represent Vitamin C? (A) Saccharic Acid (B) Gulconic Acid (C) Ascorbic Acid (D) Benzoic AcidAnswer: (C) Ascorbic Acid

Answer: (A)

Answer: (A) A – Methanol, B – Potassium Formate
7. In effective collisions the colliding molecules must have : (A) Proper orientation only (B) A certain minimum amount of activation energy. (C) Threshold energy only. (D) Threshold energy and proper orientation both.Answer: (D) Threshold energy and proper orientation both.
8. Identify the secondary amine from the given options: (A) (CH3)2CHΝΗ2 (B) CH3NHCH(CH3)2 (D) CH3(CH2)2NH2 (C) (CH3)3CNH2Answer: (B) CH3NHCH(CH3)2
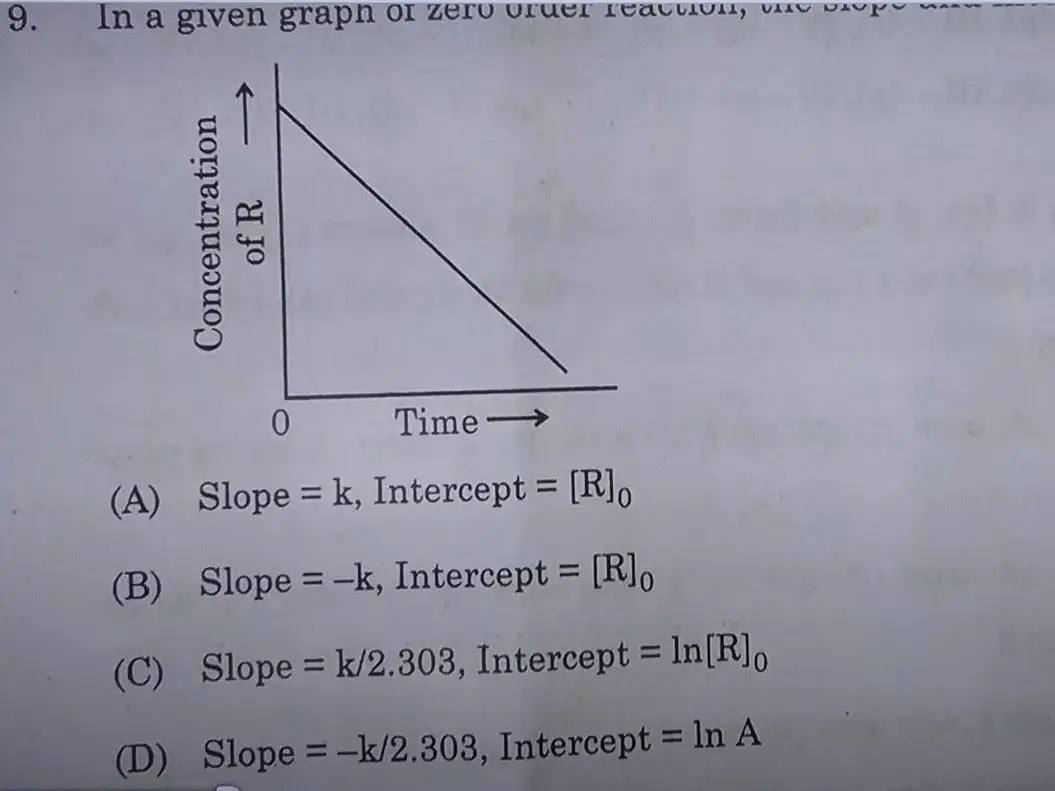
Answer: (A)
10. The general electronic configuration of d-block elements is: (A) (n-1) d1-10ns1-2 (B) (n-1) d10ns1-2 (C) (n-1) d10ns2-3 (D) (n-1) d0ns1-2Answer: (A) (n-1) d1-10ns1-2
11. Nucleophilic addition of Grignard reagent of Ketones followed by hydrolysis with dilute acids forms. (A) Alkene (B) Primary Alcohol (C) Tertiary Alcohol (D) Secondary AlcoholAnswer: (C) Tertiary Alcohol

Answer: (A)
13. Assertion (A): Bromination of Phenol can be carried out even in the absence of Lewis acid. Reason (R): – OH group of Phenol has the high activation effectAnswer: (A) both are true
14. Assertion (A): Fructose is a reducing sugar. Reason (R): Fructose does not reduce Fehling solution and Tollen’s reagent.Answer: (A) Both are True
15. Assertion (A): For a Daniell cell, Zn/Zn2+(1M) || Cu2+ (1M)/Cu Ecell = 1.1 V, if the external opposing potential is more than the electrons flow from Cu to Zn. Reason (R): Cell acts like a galvanic cellAnswer:
16. Assertion (A): Benzoic Acid does not undergo Friedel Crafts reaction Reason (R): Carboxyl group is deactivating and the catalyst aluminum chloride gets bonded to the carboxyl groupAnswer:
CBSE Class 12 Chemistry Answer Key 2024 – MCQs
| SET | Class 12 Chemistry Exam Paper 2024 Link |
|---|---|
| SET 1 | Class 12 Chemistry SET 1 Question Paper 2024 |
CBSE Class 12 Chemistry Answer Key 2024 FAQs
When will the CBSE Class 12 Chemistry Answer Key 2024 be released?
Where can I find the CBSE Class 12 Chemistry Answer Key 2024?
Are there any negative markings in the CBSE Class 12 Chemistry exam?
What is the totals marks of CBSE class 12 Chemistry Question paper?
How many Sections are there in the class 12 Chemistry Question paper?










