
Aryl Halides
Alkyl and Aryl Halides of Class 12
Aryl halides are compounds containing halogen attached directly to an benzene ring. The general formula of aryl halides is ArX, where Ar is phenyl, substituted phenyl or aryl groups.
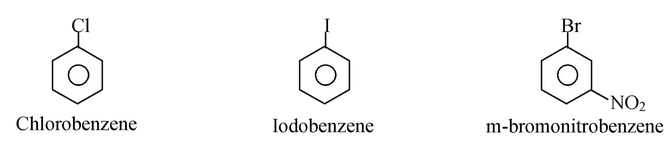
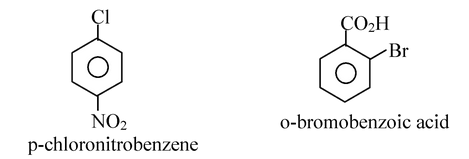
An aryl halide is not just any halogen compound containing an aromatic ring. For example, benzyl chloride is not an aryl halide, because halogen is not directly attached to the aromatic ring. Its structure and properties resemble substituted alkyl halide.
The aryl halides differ a lot from alkyl halides in their preparation and properties. Aryl halides are comparatively unreactive towards the nucleophilic substitution reactions, which are characteristic of the alkyl halides. However, the presence of certain groups on the aromatic ring greatly increases the reactivity of aryl halides towards nucleophilic substitution. In absence of such groups, reaction can still be brought about by very strongly basic reagents or under drastic conditions. Nucleophilic aromatic substitution follow two very distinct pathways.
- The bimolecular displacement mechanism−involving the carbanion as intermediate and applicable to activated aryl halides.
- The elimination addition mechanism−involving the remarkable intermediate called benzyne and applicable to unactivated and deactivated aryl halides.
Vinyl halides are the compounds in which halogen is attached directly to a doubly bonded carbon.
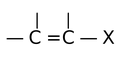
A vinyl halide show an interesting parallelism to aryl halides. Both are unreactive towards nucleophilic substitution reactions, for basically the same reason. Moreover, this low reactivity is caused partly by the partial double bond character of the carbon-halogen bond.
Structure and reactivity of aryl and vinyl halides
The low reactivity of aryl and vinyl halides towards nucleophilic displacement has been attributed to two different factors.
- delocalization of electrons by resonance and
- differences in σ bond energies due to difference in hybridization of carbon.
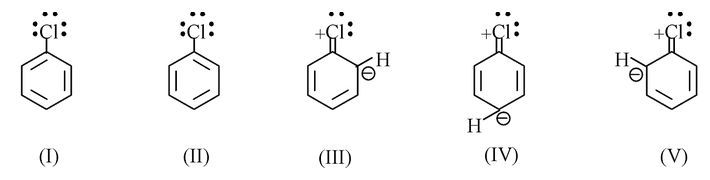
Chlorobenzene is considered to be hybrid of not only the two Kekule structures I and II, but also structures, III, IV, and V, in which chlorine is joined to carbon atom by a double bond. In structures III, IV, and V chlorine bears a positive charge and the ortho and para positions bear a negative charge.
In a similar way, vinyl chloride is considered to be a hybrid of structure VI and structure VII (in which chlorine is joined to carbon by a double bond). In (VII), chlorine bears a positive charge and C-2 bears a negative charge. Other aryl and vinyl halides have structures exactly analogous to these.
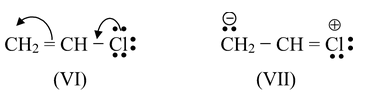
Contribution from III, IV and V, and from VII stabilizes the chlorobenzene and vinyl chloride molecules, and gives a double bond character to the carbon−chlorine bond. Carbon and chlorine are thus held together by something more than a single pair of electrons, and the carbon chlorine bond is stronger than if it were a pure single bond. The low reactivity of these halides towards nucleophilic substitution is due to resonance stabilization of the halides. This stabilization increases the Eact for displacement, and thus slows down reaction.
In alkyl halides, the carbon holding halogen atom is sp 3 hybridized. In aryl and vinyl halides, carbon is sp 2 hybridized, the carbon−halogen bond is shorter and stronger and thus the molecule is more stable.
Chlorobenzene and bromobenzene have dipole moments of only 1.7 D, and vinyl chlorides and vinyl bromides have dipole moments of only 1.4 D. This is consistant with the resonance picture of these molecules. In the structures that contain double bonded halogen (III, IV, V and VII) there is a positive charge on halogen and a negative charge on carbon, to the extent that these structures contribute to the hybrids, they tend to oppose the usual displacement of electrons towards halogen. Although there is still a net displacement of electrons towards halogen in aryl halides and in vinyl halides but it is less than other organic alkyl halides.









