
Methods Of Preparation Of Alkyl Halides
Alkyl and Aryl Halides of Class 12
(I) From Alcohols
(a) By using hydrogen halide
R − OH
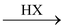 RX + H
2
O
RX + H
2
O
It must be noted that the HX used should be dry, which is produced, as follows
2NaCl + H
2
SO
4
 2HCl↑ + Na
2
SO
4
2HCl↑ + Na
2
SO
4
2NaBr + H
2
SO
4
 2HBr↑ + Na
2
SO
4
2HBr↑ + Na
2
SO
4
6NaI + 2H
3
PO
4
 6HI↑ + 2Na
3
PO
4
6HI↑ + 2Na
3
PO
4
It may be noted that H 3 PO 4 instead of H 2 SO 4 is used to prepare HI. This is because HI is a reducing agent and H 2 SO 4 is an oxidising agent.
The above substitution reactions proceed via S N 1 and S N 2 mechanism. Both the above mechanisms are operative during reaction having competition among them.
Details about S N 1 and S N 2 will be discussed later in this topic.
(b) By using phosphorous halides
ROH + PCl
5
 RCl + POCl
3
+ HCl
RCl + POCl
3
+ HCl
3ROH + PCl
3
 3RCl + H
3
PO
3
3RCl + H
3
PO
3
3ROH + PBr
3
 3RBr + H3PO3
3RBr + H3PO3
3ROH + PI
3
 3R − I + H
3
PO
3
3R − I + H
3
PO
3
Phosphorous halides are prepared by treating red phosphorous and halogen. The merit of using phosphorous halides is that free carbocation is not generated and so rearrangement does not take place.
(c) By using SOCl 2 (thionyl chloride)
R − OH + SOCl
2
 R − Cl + SO
2
↑ + HCl↑
R − Cl + SO
2
↑ + HCl↑
The reaction follows S N 2 pathway. The usefulness of this method is that there is no side product which has to be separated. The side products are gaseous SO 2 which escape from the reaction mixture and HCl which forms a salt with the base (pyridine) named pyridinium chloride (C 5 H 5 N + CL - ). In the absence of pyridine reaction follows SNi mechanism in which configuration of the alcohol is retained.
(II) By Direct halogenation of hydrocarbons
R − H
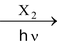 R − X + HX
R − X + HX
Reactivity of above reaction with respect to type of hydrogen to be replaced follows following order
Tertiary hydrogen > Secondary hydrogen > Primary hydrogen
As far as the reactivity of halogen is concerned, F2 is most reactive while I2 is least reactive. Infact reaction with I2 is reversible and is carried out in the presence of some oxidising agents like HIO 3 , HNO 3 etc. to oxidise HI. Mechanisms of these reactions has been discussed in the topic “alkanes”.
(III) By halide Exchange (Finkelstein reaction)
Alkyl iodides can be prepared from alkyl chlorides and alkyl bromides by nucleophilic substitution. This can be achieved by treating them with NaI, using acetone as a solvent. Feasibility of this reaction is due to the solubility of NaI in acetone and more nucleophilic character of I− ion. It follows SN2 mechanism.
R − Cl
 R − I + NaCl
R − I + NaCl
R − Br
 R − I + NaBr
R − I + NaBr
(IV) By addition of HX to alkenes
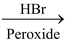
Alkyl chlorides, bromides and iodides can be prepared by treating an alkene with corresponding hydrogen halides (HCl, HBr and HI). The addition of these compounds to alkene takes place according to Markovnikov's rule as discussed earlier in the topic “alkenes”. The reaction proceeds by electrophilic addition of H + to give more stable carbocation followed by attack of X−. Anti−Markovnikov addition of HBr can be achieved if reaction is carried out in presence of peroxide (H 2 O 2 or benzoyl peroxide). This reaction proceeds by the attack of bromine free radicals (.Br).
CH
3
− CH = CH
2
 CH
3
−
CH
3
−
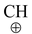 − CH
3
− CH
3
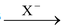
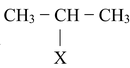
CH
3
− CH = CH
2
 CH
3
−
CH
3
−
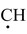 − CH
2
−Br
− CH
2
−Br
 CH
3
− CH
2
− CH
2
Br + Br∙
CH
3
− CH
2
− CH
2
Br + Br∙
(V) From silver salts of carboxylic Acid
RCOOAg + X2
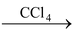 RX + AgX + CO
2
RX + AgX + CO
2
(X 2 = Cl 2 or Br 2 )
This reaction is popularly known as Hunsdieker reaction.
Mechanism
The mechanism is uncertain but probably in the first step acyl hypohalite is formed which then decomposes into free radicals.
RCOO
2
Ag + X
2
 RCOOX + AgX
RCOOX + AgX
RCOOX
 RCOO∙ + X∙
RCOO∙ + X∙
RCOO∙
 R∙ + CO
2
R∙ + CO
2
R∙ + X
2
 RX + X∙
RX + X∙
R∙ + RCOOX
 RX + RCOO∙ etc.
RX + RCOO∙ etc.
In Hunsdieker reaction, Br 2 in CCl 4 gives better yield than Cl 2 in CCl 4 .
The yield and ease of formation of R −X is
1°RX > 2°RX > 3°RX
If I2 is used in Hunsdieker reaction in CCl 4 , then an ester is formed. This is known as Birnbaum−Simonini Reaction.
(VI) Preparation of allylic or benzylic halides
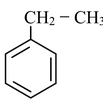
(i) Direct halogenation of any aromatic hydrocarbon preferably gives benzylic halide. This is because benzyl radical is resonance stabilized.
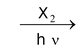
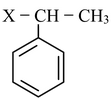
(ii) If we treat alkene with halogen at high temperature or in presence of radiations or any reagent which is able to provide halogen radicals in low concentrations, then allyl halides are formed.
CH3 − CH = CH2
 X − CH2 − CH = CH2
X − CH2 − CH = CH2
CH
3
− CH = CH
2
+ Cl
2

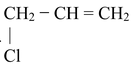
CH
3
− CH = CH
2

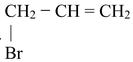
CH
3
− CH = CH
2

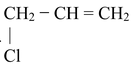
(VII) Preparation of Polyhalides
(i) Addition of halogen to alkenes produces vicinal dihalides.
CH
2
= CH
2
+ X
2
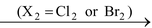
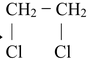
(ii) Addition of halogen (Cl 2 or Br 2 ) to alkynes produces tetrahalodervatives.
HC ≡ CH
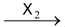
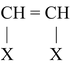
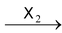
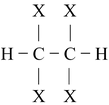
Mechanism of addition of halogen to alkenes has been discussed in the topic “alkenes”.
(VIII) Preparation of Vinyl halides
CH ≡ CH
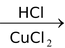
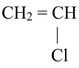
The above reaction proceeds via electrophilic addition as follows
CH ≡ CH
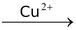
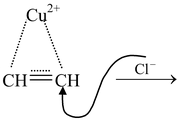
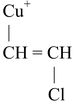
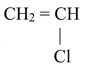
Also Check









