
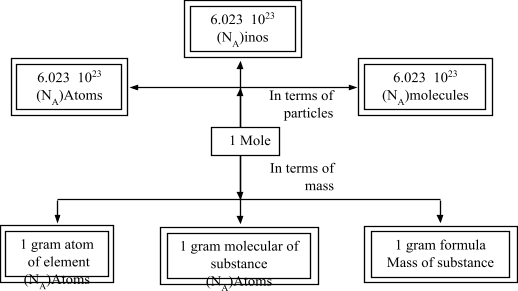
Important Formula of Atom and Molecules
Atom and Molecule of Class 9
Some Useful Formulas
-
Number of moles of atoms =
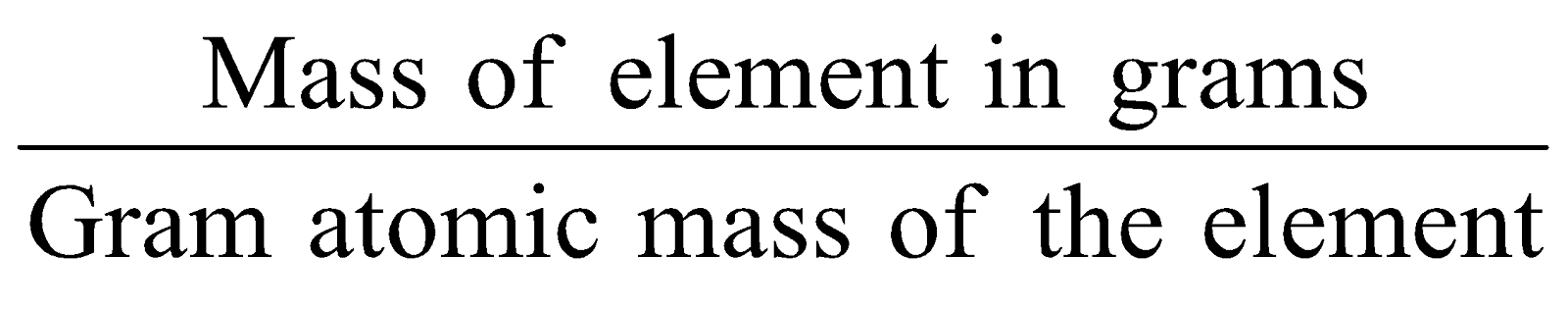
-
Number of moles =
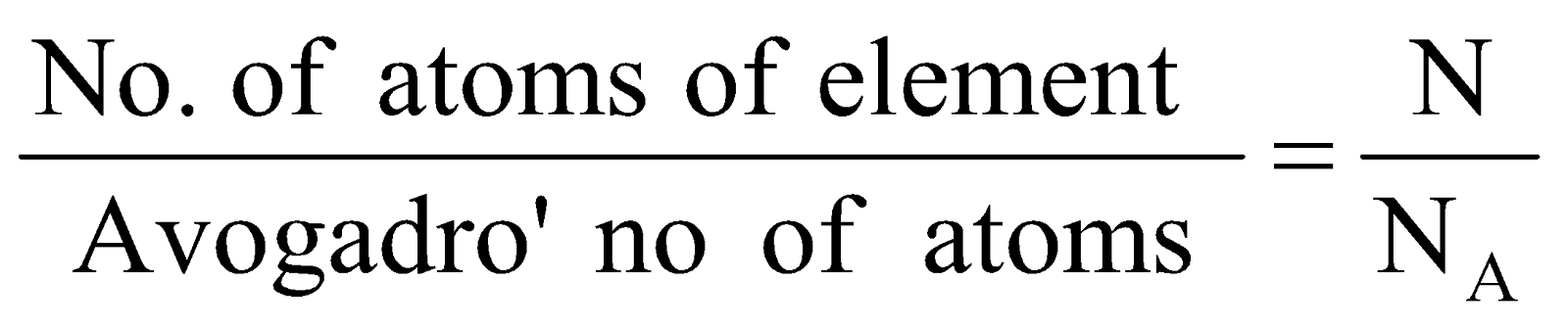
-
Number of moles of molecules =
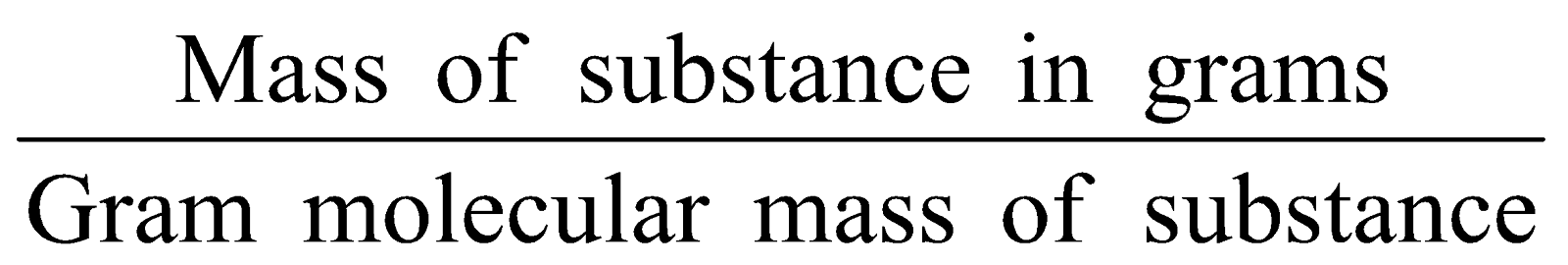
-
Number of moles of molecules =
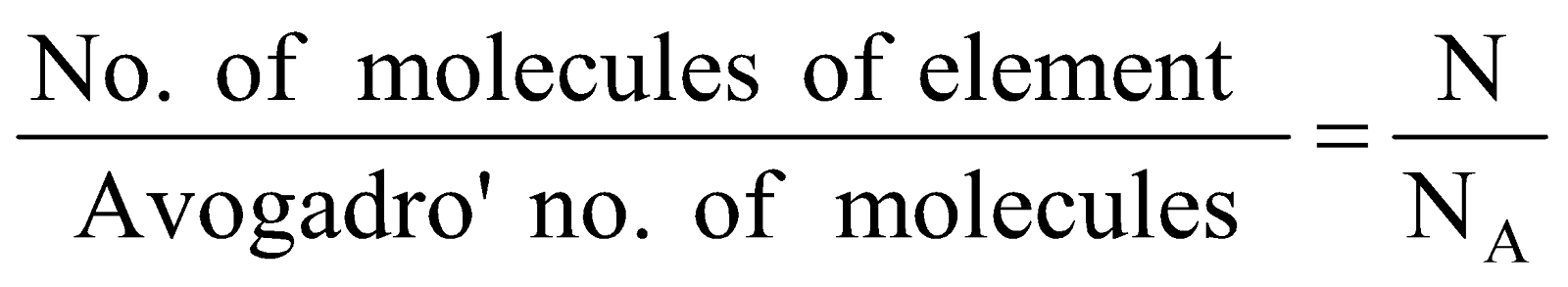
- 1 mole of molecules = Gram molecular mass
- 1 mole of molecules = 6.023 × 10 23 molecules
Ex. Calculate the mass of the following:
- 0.5 mole of O 2 gas
- 0.5 mole of O atoms
- 3.011 × 10 23 atoms of O
- 6.023 × 10 23 molecules of O 2 .
(Given : Gram atomic mass of oxygen = 16 g, gram molecular mass of oxygen (O 2 ) = 32 g).
Solution:
- 0.5 mole of O2 gas
No. of moles =
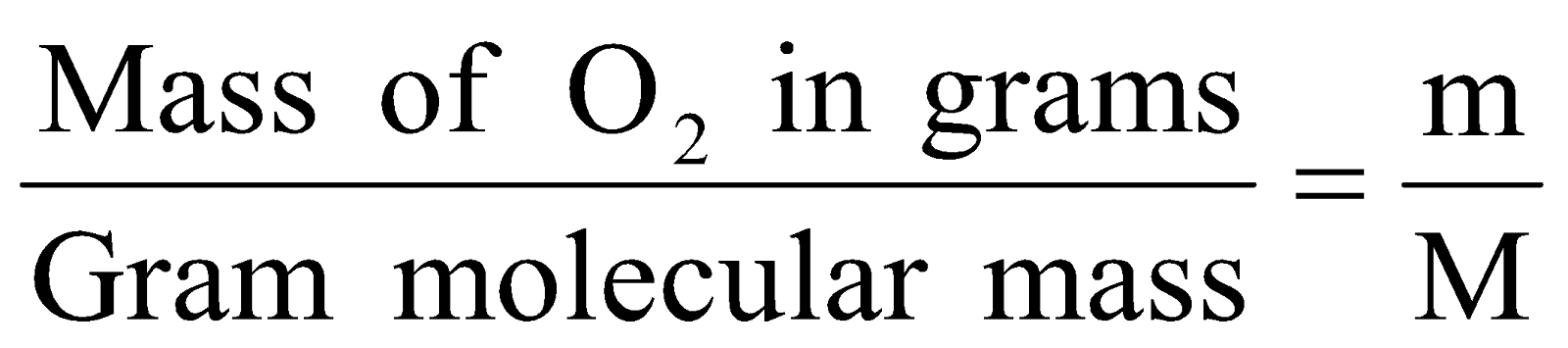
 Mass of O
2
in grams (m) = No. of moles × M
Mass of O
2
in grams (m) = No. of moles × M
= 0.5 × (32g) = 16g
- 0.5 mole of oxygen (O) atoms
No. of moles =
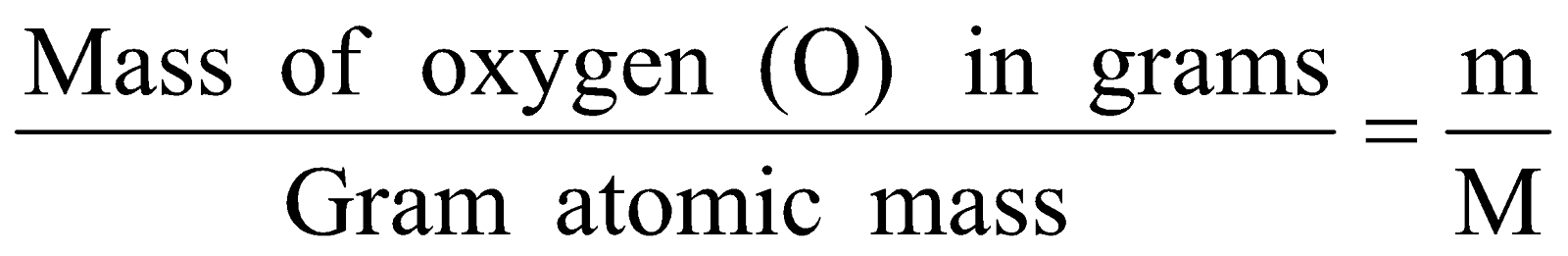
Mass of oxygen (O) in grams (m) = No. of moles × M
= 0.5 × (16g) = 8g.
- 3.011 × 10 23 atoms of oxygen (O)
Step I : Calculation of no. of gram atoms of oxygen
No. of gram atoms =
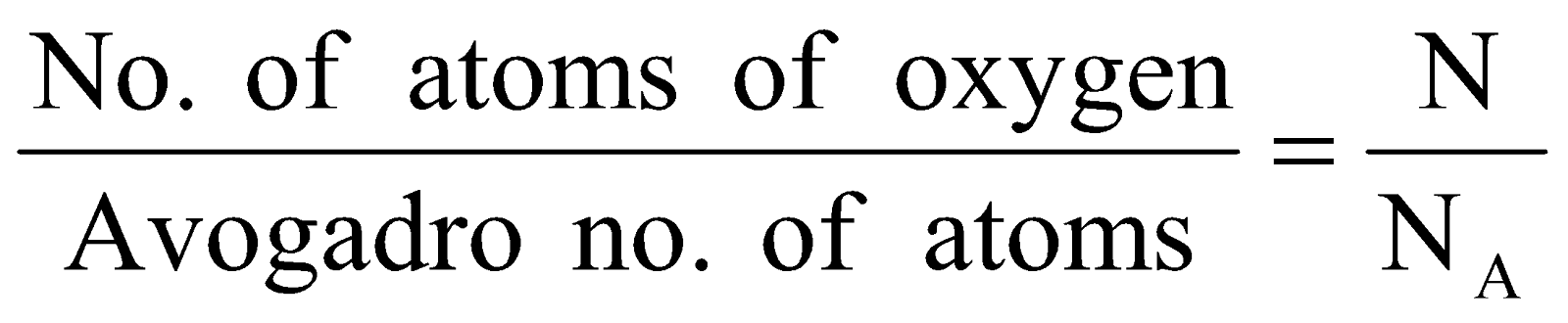
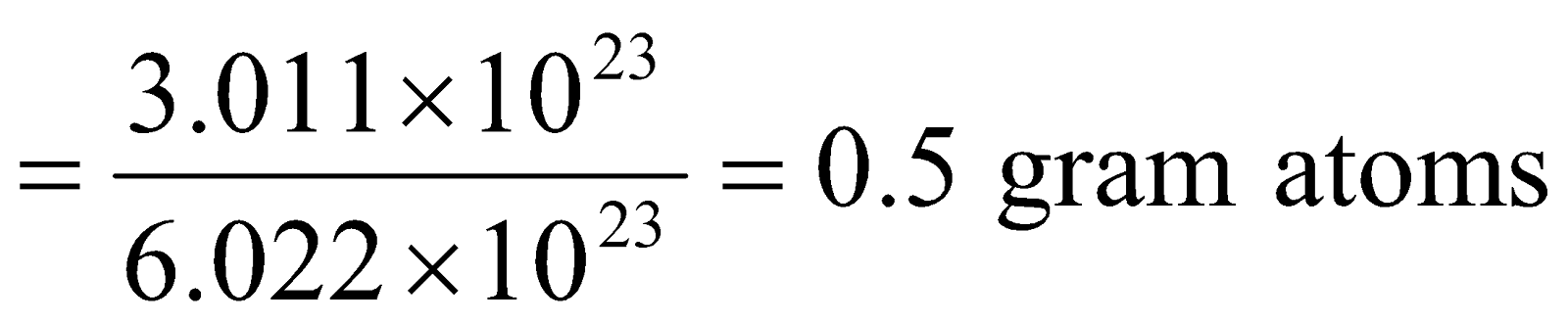
Step II: Calculation of mass of oxygen (O) atoms
Mass of oxygen (O) atoms = Gram atomic mass of oxygen x No. of gram atoms of oxygen.
= 16 × 0.5 = 8g.
- 6.023 × 1023 molecules of oxygen (O2) -
Step I : Calculation of no. of moles of oxygen.
No. of moles =
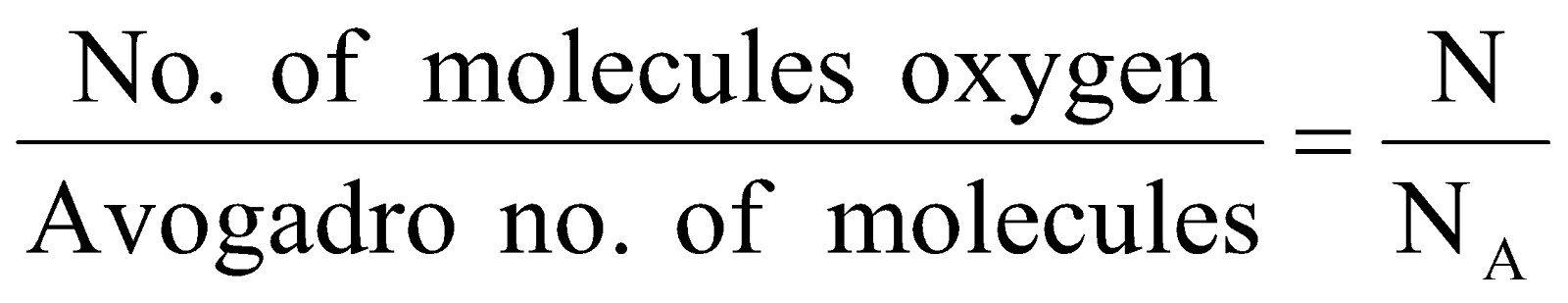
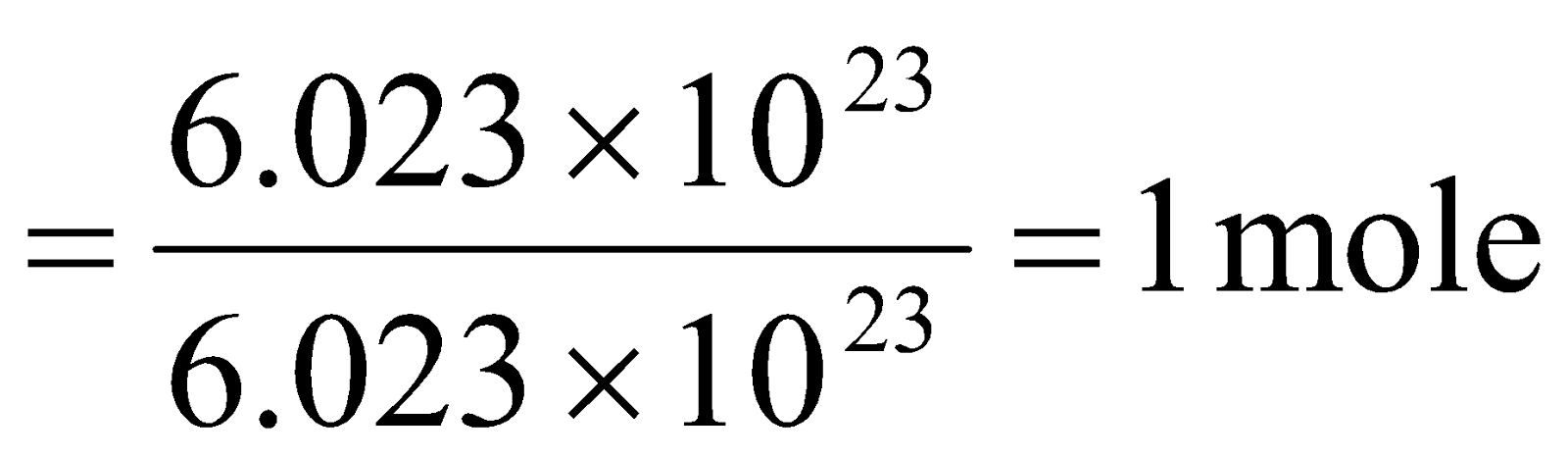
Step II : Calculation of mass of oxygen (O2) molecules.
Mass of oxygen (O2) molecules = Gram molecular mass of oxygen x No. of moles of oxygen.
= 32 × 1 = 32 g
Frequently Asked Question (FAQs)
Q1. What are atom and molecule class 9?
Ans. An atom is the smallest particle of matter that takes part in a chemical reaction.
Q2. What is the size of atom Class 9?
Ans. They are tiny, and their radius can be measured in nanometers. Atomic radius is measured in nanometers. 1/109 m = 1 nm or 1m = 10 9 nm.
Q3. What are the four types of atoms?
Ans. So… what makes atoms different from one another? Well, let's look at hydrogen, oxygen, carbon, and nitrogen, the four most common elements in the solar system.
Q4. What is the size of a molecule?
Ans. Molecules commonly used as building blocks of organic synthesis have a magnitude of a few angstroms (Å) to a few zini ounces or around half a million meters.
Q5. What is the shape of an atom?
Ans. Atoms do not have a well-defined outer boundary, so the atom's position usually defines its size. This measures the distance an electron cloud emanates from the nucleus. This assumes that the atom exhibits a spherical shape, which is only heard of in atoms in space or free space.
Q6. What are atoms made of?
Ans. Atoms are made up of two fundamental particles: electrons and quarks. Electrons take up space around the nucleus of an atom. Each electron has 1 electric charge. Quarks make protons and neutrons, which, in turn, form the nucleus of an atom.
Q7. Who discovered the electron?
Ans. J.J. Thomson discovered the electron. He was a professor of physics at the University of Cambridge in the UK. He placed cathode tubes in electrical and magnetic fields.
Q8. What are five examples of molecules?
Ans. Here are examples of common molecules:
- H 2 O (water)
- N 2 (nitrogen)
- O 3 (ozone)
- Cao (calcium oxide)
- C 6 H 12 O 6 (glucose, a type of sugar)
- NaCl (table salt)
Q9. What is the smallest atom?
Ans. The smallest atom is helium with a radius of 31 pm, while the largest is cesium at 298 pm. However, hydrogen has a lower atomic number than helium; the calculated radius of the hydrogen atom is about 70% larger.
Also Check









