
Introduction
Introduction to organic chemistry of Class 9
The word Organic is one of the most overused in the English language.
People use it as a derogatory term in phrases like Don’t eat that; it’s not organic. Of course, there is a precise scientific definition of the word. In science, organic can be a biological or chemical term. In Biology it mean any thing that is living or has lived. The opposite is Non-Organic. In Chemistry, an organic compound is one containing Carbon atoms. The opposite term is Inorganic.
VITAL FORCE THEORY OR BERZELIUS HYPOTHESIS
Organic compounds cannot be synthesized in the laboratory because they require the presence of a mysterious force (called vital force) which exists only in the living organisms.
Wholer’s synthesis: When ammonium cyanate, obtained by double decomposition of ammonium chloride and potassium cyanate is heated, urea is formed. After this synthesis vital force theory was rejected.
NH4Cl + KCNO
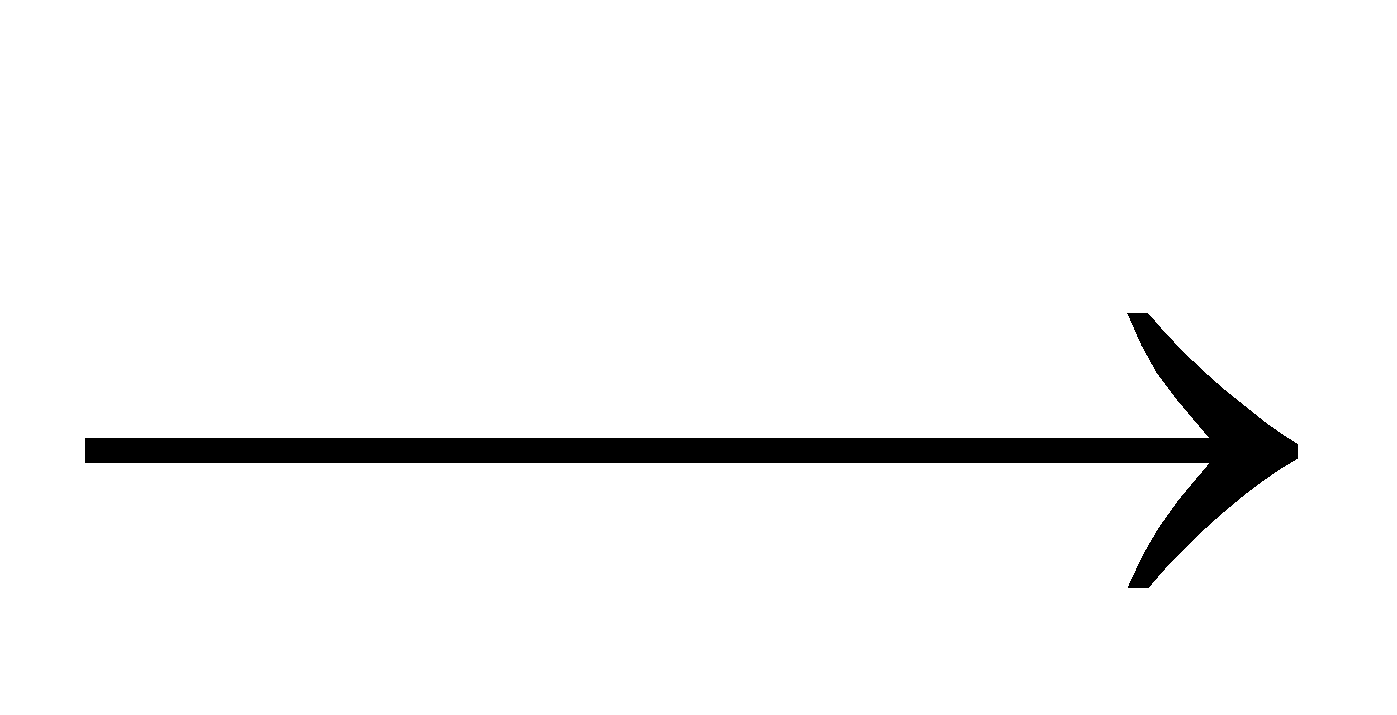 NH4CNO + KCl ; NH4CNO
NH4CNO + KCl ; NH4CNO
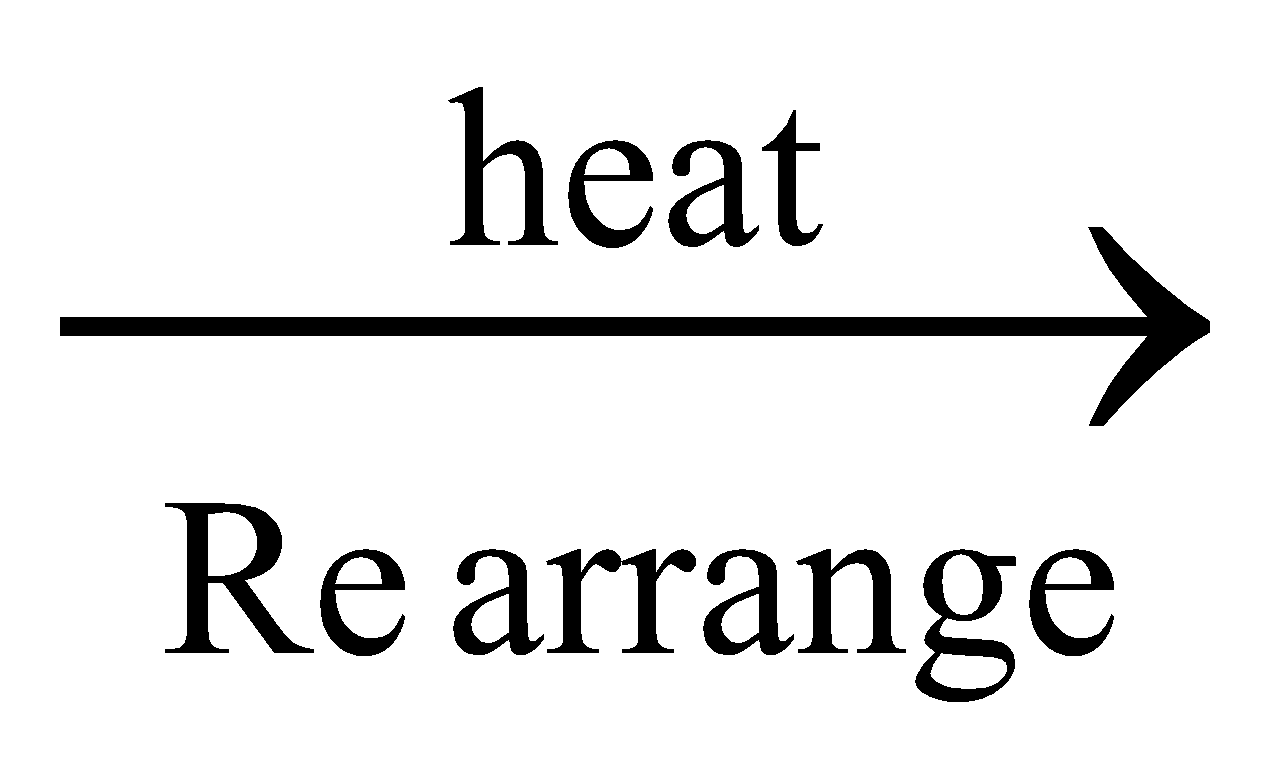 NH2CONH2
NH2CONH2
Urea
Characteristics of organic compounds
- are made from only a few elements
- are larger in number
- have complex structures,
- contain covalent and co−ordinate bonds,
- are soluble in non−polar solvents,
- have low melting and boiling points,
- are bad conductors of electricity,
- undergo molecular reactions which are generally slow and reversible,
- show isomerism and
- exhibit homology.
Electronegativity and strength of bonds: The electronegativity of carbon is close to a number of other elements such as hydrogen, nitrogen, phosphorus, chlorine, oxygen. As a result, carbon forms strong covalent bonds with these elements as well. Tendency to form multiple bonds: Because of its small size, carbon has a strong tendency to form multiple (double and triple) bonds with carbon, oxygen and nitrogen atoms.
Isomerism: Many organic compounds show the phenomenon of isomerism by virtue of which a single molecular formula may represent two or more structures.
NCERT solutions for class 9 Science prepared by Physics Wallah will help you to solve your NCERT text book exercise.









