
Chemical Properties Of Metals
Metal And Non-metals of Class 10
CHEMICAL PROPERTIES OF METALS
Metals are highly electropositive elements, i.e. they form positive ions by losing electrons.
M → M n+ + ne –
For example:
Na → Na + + e –
Mg → Mg 2+ + 2e –
Metals react with oxygen, water, dilute acids, and other salts. The characteristic chemical properties of metals are due to their electropositive character.
1. Metals are Electropositive : Atoms of metal elements have 1 to 3 electrons in the outermost shells. The atoms can therefore loose electrons while forming compounds. The metal atoms become electropositive in a reaction. For example, Na atom easily gives off its one electron in the M-shell to become Na + .
2. Reaction with Oxygen : All metals react with oxygen to form metal oxides. A single metal can form various oxides depending on the valence state of the metal atom. For example Fe can form oxides FeO, Fe2O3, Fe 3 O 4 , etc. Metal oxides are basic in nature; when dissolved in water they form alkaline solution. Metal oxide bonds are ionic bonds. Metal atoms loose electrons to oxygen atoms while forming the metal oxide. Not all metals react with oxygen at room temperature. Some metals need to be held at high temperatures before they start reacting with oxygen.
To express the order of reactivity of the metals with oxygen, we can say that
Na > Mg > Zn > Fe > Cu
(i) Sodium and potassium react with oxygen at room temperature to form basic oxides.
4Na + O 2 → 2Na 2 O
Sodium Oxygen Sodium oxide
4K + O 2 → 2K 2 O
Potassium Oxygen Potassium oxide
Most of the metal oxides are insoluble in water. But, some of the metal oxides dissolve in water to form alkalis.
Sodium and potassium oxide are the two metal oxides which are soluble in water and form alkalis.
Na 2 O + H 2 O → 2NaOH
Sodium oxide Sodium hydroxide
(Basic oxide) (An alkali)
K2O + H 2 O → 2KOH
Potassium oxide Potassium hydroxide
(Basic oxide) (An alkali)
(ii) Magnesium does not react with oxygen at room temperature. It combines with oxygen only on heating. On heating, magnesium burns in air producing intense heat and light.
2Mg + O 2 → 2MgO
Magnesium Oxygen Magnesium oxide
Since reaction of magnesium with oxygen takes place at higher temperature than the reaction of sodium with oxygen, it means magnesium is less reactive than sodium.
(iii) Zinc metal burns in air only on strong heating to form zinc oxide.
2Zn + O 2 → 2ZnO
Zinc Oxygen Zinc oxide
(iv) Iron does not burn in air even on strong heating. The reaction of iron with oxygen takes place less readily than that of zinc.
2Fe + O 2 → 2Fe 3 O 4
Iron Oxygen Iron oxide
Fe 3 O 4 is a mixture of ferrous oxide (FeO) and ferric oxide (Fe 2 O 3 ) and is known as iron (II, III) oxide.
(v) Copper is very less reactive towards oxygen. Copper does not burn, but the hot metal is coated with a black coloured layer of copper(II) oxide.
2Cu + O 2 → 2CuO
Copper Oxygen Copper(II) oxide
So, the order of reactivity of these metals with oxygen is:
Na > Mg > Zn > Fe > Cu
Silver and gold do not react with oxygen even at high temperature.
Amphoteric oxides
Though most of the metal oxides are basic in nature but some of them show basic as well as acidic nature. Those metal oxides which show basic as well as acidic behaviour are known as amphoteric oxides. Aluminium metal and zinc metal forms amphoteric oxides. Amphoteric oxides react with both, acids as well as bases to form salt and water.
(i) Aluminium oxide as basic oxide: Aluminium oxide reacts with hydrochloric acid to form aluminium chloride and water.
Al 2 O 3 + 6HCl → 2AlCl 3 + H 2 O
Aluminium oxide Hydrochloric acid Aluminium chloride Water
(Acid) (Salt)
(ii) Aluminium oxide as acidic oxide: Aluminium oxide reacts with sodium hydroxide to form sodium aluminate and water.
Al 2 O 3 + 2NaOH → 2NaAlO 2 + H 2 O
Aluminium oxide Sodium Hydroxide Sodium aluminate Water
(Base) (Salt)
Zinc oxide is an amphoteric oxide which reacts with acids as well as with bases to form salt and water. (iii) Zinc oxide as basic oxide: Zinc oxide reacts with hydrochloric acid to form zinc chloride (salt) and water.
ZnO + 2HCl → ZnCl 2 + H 2 O
Zinc oxide Hydrochloric acid Zinc chloride Water
(Acid) (Salt)
(iv) Zinc oxide as acidic oxide: It reacts with sodium hydroxide to form sodium zincate and water.
ZnO + 2NaOH → Na 2 ZnO 2 + H 2 O
Zinc oxide Sodium hydroxide Sodium zincate Water
(Base) (Salt)
3. Metal Reaction with Water : All metals react with water to form metal oxides and metal hydroxides. Hydrogen gas is released in this reaction. Metal oxides are basic in nature; when dissolved in water they form alkaline solution. Not all metals react with water at room temperature. Some metals react with cold water, some metals react with hot water, and other metals react with steam. There are some metals that do not react with water at all. These reactions will become more clear in the following discussion.
(i) Metals such as potassium, sodium and calcium react with cold water.
2K + 2H 2 O → 2KOH + H 2
Potassium Water Potassium hydroxide
2Na + 2H 2 O → 2NaOH + H 2
Sodium Water Sodium hydroxide
Ca + 2H 2 O → Ca(OH) 2 + H 2
Calcium Water Calcium hydroxide
Potassium decomposes water more vigorously than sodium. Hence, potassium is more reactive than sodium. Sodium decomposes water more vigorously than calcium. Hence, sodium is more reactive than calcium.
(ii) Magnesium does not react with cold water. It reacts with hot water to form magnesium hydroxide and hydrogen.
Mg + 2H 2 O → Mg(OH) 2 + H 2
Magnesium Water Magnesium hydroxide
(hot)
(iii) Metals like aluminium, iron and zinc do not react either with cold or hot water. They react with steam to form metal oxide and hydrogen.
2Al + 2H 2 O → Al 2 O 3 + 3H 2
Aluminium Steam Aluminium oxide
Zn + H 2 O → ZnO + H 2
Zinc Steam Zinc oxide
3Fe + 4H 2 O → Fe 3 O 4 + 4H 2
Iron Steam Iron(II, III) oxide
(Red hot)
The metals such as lead, copper, silver and gold do not react with water at all.
Copper metal does not react with water or steam. This shows that Cu is less reactive than Fe.
Thus in terms of reactivity of metals with water, we can say that Na is the most reactive metal and Cu is the least reactive of the examples that we have seen above.
To express the order of reactivity of the metals with water, we can say that
Na > Mg > Zn > Fe > Cu
Metals evolve hydrogen from water
Water is a very weak electrolyte. It ionizes to give hydrogen ions (H + ) and hydroxide ions (OH – ).
H 2 O → H + + OH –
When a metal reacts with water, it loses electrons to reduce H + ions to H atoms.
2H + + 2e – → H 2
The H atom then unites to give H 2 gas.
4. Metal reaction with dilute acids :
Most metals react with dilute acids by replacing hydrogen and forming a salt. Hydrogen gas is released. Metals like Cu, Ag, Au, Pt, Hg, do not react withdilute acids. The rate of reaction depends on the reactivity of the metal. Na reacts vigorously with dilute acids, whereas Zn or Fe react very slowly with dilute acids.
With dilute hydrochloric acids, metals react to give metal chloride and hydrogen. Some examples are given below.
Reaction of metals with hydrochloric acid
(i) Sodium metal reacts with dilute hydrochloric acid vigorously to form sodium chloride and hydrogen gas.
2Na + 2HCl → 2NaCl + H 2
Sodium chloride
This shows that sodium is a very reactive metal.
(ii) Magnesium reacts rapidly with dilute hydrochloric acid to form magnesium chloride and hydrogen gas.
Mg + 2HCl → MgCl 2 + H 2
Magnesium chloride
(iii) Aluminium metal reacts rapidly with dilute hydrochloric acid to form aluminium chloride and hydrogen gas.
2Al + 6HCl → 2AlCl 3 + 3H 2
Aluminium chloride
At first, it reacts slowly due to the presence of tough protective layer of aluminium oxide on its surface. But when the outer oxide layer gets dissolved in acid, then fresh aluminium metal is exposed which reacts rapidly.
(iv) Zinc reacts with dilute hydrochloric acid to produce zinc chloride and hydrogen but less rapidly than that of aluminium.
Zn + 2HCl → ZnCl 2 + H 2
Zinc chloride
This reaction shows that zinc is less reactive than aluminium.
(v) Iron reacts slowly with dilute hydrochloric acid to form ferrous chloride
Fe + 2HCl → FeCl 2 + H 2
Ferrous chloride
(vi) Copper does not react with dilute hydrochloric acid.
Cu + HCl → No reaction
Gold and silver metal also do not react with dilute acids.
With dilute sulphuric acids, metals react to give metal sulphate and hydrogen. Some examples are given below.
Reaction of a metal with dilute sulphuric acid
Metals displace hydrogen from dilute sulphuric acid. For example :
2Na (s) + H 2 SO4 (aq) → Na 2 SO 4 (aq) + H 2 (g)
Sodium Sulphuric acid Sodium sulphate Hydrogen
Mg (s) + H 2 SO 4 (aq) → MgSO 4 (aq) + H 2 (g)
Magnesium Sulphuric acid Magnesium sulphate Hydrogen
2Al (s) + 3H 2 SO 4 (aq) → Al 2 (SO 4 ) 3 (aq) + 3H 2 (g)
Aluminium Sulphuric acid Aluminium sulphate Hydrogen
Zn (s) + H 2 SO 4 (aq) → ZnSO 4 (aq) + H 2 (g)
Zinc Sulphuric acid Zinc sulphate Hydrogen
Cu (s) + H 2 SO 4 (aq) → No reaction
Copper Sulphuric acid
Reactions with dilute nitric acids and metals are not as straight forward as the reactions shown above. Nitric acid is an oxidizing acid and releases O from its NO3—radical, hence its reactions with metals does not lead to metal-salt and hydrogen.
Very dilute nitric acid, however, reacts with magnesium and manganese metals to evolve hydrogen gas. This is because very dilute nitric acid is a weak oxidizing agent which is not able to oxidize hydrogen to water.
(i) Magnesium reacts with very dilute nitric acid to form magnesium nitrate and hydrogen gas.
Mg(s) + 2HNO 3 (aq) → Mg(NO 3 ) 2 (aq) + H 2 (g)
Magnesium Nitric acid Magnesium nitrate Hydrogen
(ii) Manganese reacts with very dilute nitric acid to form manganese nitrate and hydrogen gas.
Mn (s) + 2HNO 3 (aq) → Mn(NO 3 ) 2 (aq) + H 2 (g)
Manganese Nitric acid Manganese nitrate Hydrogen
From the dilute acid reactions, we can conclude that Na is more reacts most rapidly and Cu does not react at all. So the order of reactivity of these metals with dilute acids can be written as
To express the order of reactivity of the metals with dilute acids, we can say that
Na > Mg > Zn > Fe > Cu
5. Metal reactions with salt solutions:
When a more reactive metal is placed in reactive metal from its salt solution of less reactive metal, then the more reactive metal displaces the less reactive metal from its salt solution. For example, we will take a solution of copper sulphate (blue coloured solution) and put a strip of zinc metal in the solution. It is observed that the blue colour of copper sulphate fades gradually and copper metal is deposited on the zinc strip. This means that the following reaction occurs:
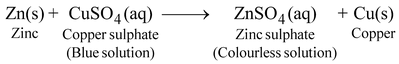
Here, zinc displaces copper from its salt solution.
However, if we take zinc sulphate solution and put a strip of copper metal in this solution, no reaction occurs.
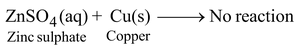
This means that copper cannot displace zinc metal from its solution. Thus, we can conclude that zinc is more reactive than copper. However, if we put gold or platinum strip in the copper sulphate solution, then copper is not displaced by gold or platinum. Thus, gold and platinum are less reactive than copper.
6. Metal reactions with metal oxides:
Reactivity of metals determine which metal displaces another metal from the metal-oxide. As discussed earlier, more reactive metal displaces the less reactive metal in the metal oxide.
When CuO is heated with Mg, MgO is formed and Cu is released. This is because Mg is more reactive than Cu. This reaction is irreversible, as Cu will never be able to displace Mg from MgO.
(i) Aluminium can displace iron from ferric oxide.
2Al + Fe 2 O 3 → Al 2 O 3 + 2Fe
Aluminium Ferric oxide Aluminium oxide Iron
(ii) Magnesium can displace copper from copper oxide.
Mg + CuO → MgO + Cu
Magnesium Copper (II) oxide Magnesium oxide Copper
7. Metal reactions with chloride :
Metals have excess electrons and chlorine (Cl) needs one electron to complete its orbit. Hence metals react with Cl easily. Na, Ca react readily with Cl to form NaCl and CaCl2. Mg reacts with Cl on heating to form MgCl2. Zn forms a chloride easily. Fe and Cu need to be heated to form their chlorides. All chlorides are electrovalent or ionic compounds. The reactions are shown below.
(i) Sodium readily reacts with chlorine to form an electrovalent chloride called sodium chloride.
2Na + Cl 2 → 2NaCl
Sodium Chlorine Sodium chloride
(ii) Calcium reacts vigorously with chlorine to form an electrovalent chloride called calcium chloride.
Ca + Cl 2 → CaCl 2
Calcium Chlorine Calcium chloride
(iii) Magnesium on heating forms magnesium chloride, which is an electrovalent chloride.
Mg + Cl
2
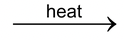 MgCl
2
MgCl
2
Magnesium Chloride Magnesium chloride
(iv) Zinc combines directly with chlorine to form zinc chloride.
Zn + Cl 2 → ZnCl 2
Zinc Chloride Zinc chloride
(v) Iron combines with chlorine, when heated, to form iron (III) chloride or ferric chloride
2 Fe + 3Cl
2
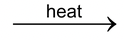 2 FeCl
3
2 FeCl
3
Iron Chlorine Ferric Chloride
(vi) On heating, copper reacts with chlorine to form copper (II) chloride or cupric chloride
Cu + Cl 2 → CuCl 2
Copper Chlorine Cupric chloride
8. Metal reactions with hydrogen :
Na, K, Mg and Ca react with H to form metal-hydrides. Other metals do not react with H easily. To form metal-hydrides, the metals have to be heated and hydrogen gas has to be passed over the heated metal fillings. Metals have to readily give up their electron to the hydrogen to form the metal-hydrogen bond. NaH, KH, CaH 2 and MgH 2 are common metal-hydrides. Copper (Cu), iron (Fe) and zinc (Zn) are less reactive metals are they do not react with hydrogen and their metal-hydrides are not formed, even when heated.
(i) When hydrogen gas is passed over heated sodium, then sodium hydride is formed.
2Na + H 2 → 2NaH
Sodium Sodium hydride
(ii) When hydrogen gas is passed over heated potassium, then potassium hydride is formed.
2K + H 2 → 2KH
Potassium Potassium hydride
(iii) When hydrogen gas is passed over heated calcium, then calcium hydride is formed.
Ca + H 2 → CaH 2
Calcium Calcium hydride
The comparatively less reactive metals like zinc, copper and iron do not react with hydrogen.
9. Metals as reducing agents :
Metals have excess unbound electrons and are able to give these electrons easily. In chemistry this property of a substance to donate electrons is known as reducing property and the substance is called a reducing agent. For example Na gives off an electron to any other substance which is forming a bond with itself. Thus Na is a reducing agent. Na atoms becomes ionized, hence Na itself is getting oxidized.
Metals have been arranged in decreasing order of their activities (or reactivities) in the activity series. After performing displacement experiments, the following series known as the reactivity or activity series has been developed as follows :
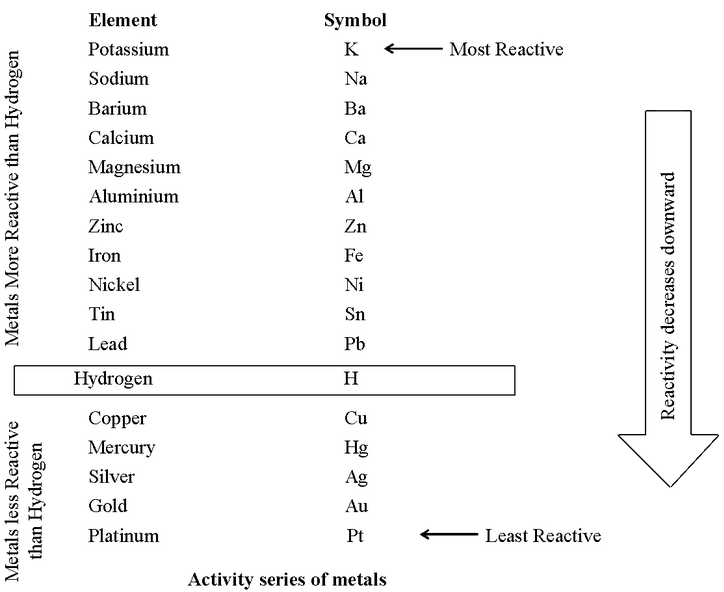
The metals above hydrogen in the activity series have greater tendency than hydrogen to give up electrons in their solutions. Such metals are called electropositive metals.
The electropositive character of metals becomes less pronounced as we go down the series. For example, potassium (K), the first metal in the series is the most electropositive, while platinum (Pt), the last metal of the series is the least electropositive.
(ii) The metals above hydrogen in the series can liberate hydrogen when treated with an acid solution. Thus, magnesium and zinc react with dilute solutions of sulphuric acid to produce hydrogen gas.
Mg + H 2 SO 4 → MgSO 4 + H 2
Zn + H 2 SO4 → ZnSO 4 + H 2
In these reactions, electrons released by metals are accepted by H + or H 3 O + ions present in the acid solution.
Mg → Mg 2+ + 2e –
2H + + 2e − → H2
or 2H 3 O + + 2e – → 2H 2 O + H 2
Thus, H + (or H 3 O + ) ions act as oxidizing agents while the metal acts as reducing agent.
(iii) A more electropositive metal can replace a less electropositive metal from the solution of a salt of the less electropositive metal. For example, when an iron rod is dipped into a solution of copper sulphate, reddish coloured copper is deposited on the iron rod.
Fe + CuSO 4 → FeSO 4 + Cu
This is because iron is more electropositive than copper









