
Chemical kinetics is the study of the rate at which chemical reactions occur. It helps us understand the factors affecting these rates, the mechanism of the reaction, and the sequence of events that lead to the formation of products.
Rate of a Chemical Reaction
Rate of Reaction is the change in concentration of reactants or products per unit time.
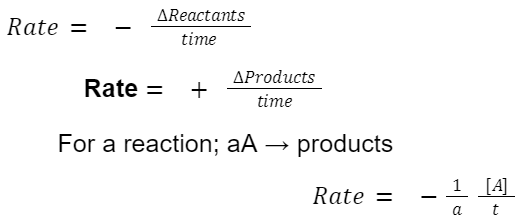
Where: [A] is the concentration of reactant A.
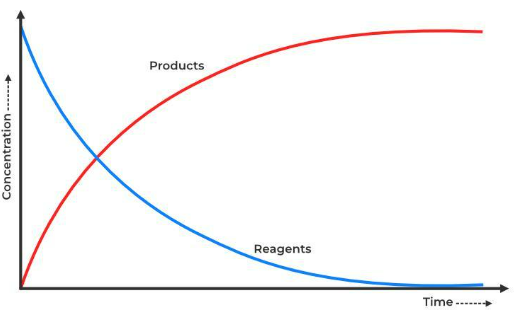
a is the stoichiometric coefficient of A in the balanced chemical equation. Graphically, the rate is represented by the negative of slope of the concentration vs. time graph for reactants, and the positive slope for products.
Factors Affecting Rate of Reaction
Concentration of Reactants: Rate usually increases with increasing concentration.
Temperature: Typically, rate increases with temperature.
Presence of Catalyst: Catalysts can increase the rate.
Surface Area: Especially in solid reactions, greater surface area can lead to faster rates.
Pressure: Especially for gaseous reactions, changes in pressure can affect the rate.
Order of Reaction
The order of a reaction indicates the power to which the concentration term is raised in the rate equation.
For a reaction:
aA + bB → Products
Rate α [A] m [B] n
Rate = k[A] m [B] n
Where m and n are orders with respect to A and B respectively.
The overall order is m+n.
Molecularity of Reaction
Molecularity refers to the number of molecules taking part in an elementary reaction step.
Molecularity can be maximum of 3.
Also Check – Gibbs Free Energy Formula
Integrated Rate Equations
Zero Order Reaction
A → Products
Rate = - d[R]/dt
d[R] =-k [dt]
[R] = -kt +[R] 0
Where [R] 0 =1
Graph: ln[R] vs. ln t is linear.
![ln[R] vs. ln t is linear.](https://static.pw.live/5eb393ee95fab7468a79d189/GLOBAL_CMS_BLOGS/856c3c00-a68b-4e96-bf15-a2cd7b4390fd.png)
Half-Life Zero Order: t 1/2 = 2k [A] 0
Example: Decomposition of NH₃ on a platinum surface.
First Order Reaction
A → Products
ln[R] = ln[R] 0 − kt
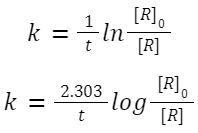
Graph: is linear.
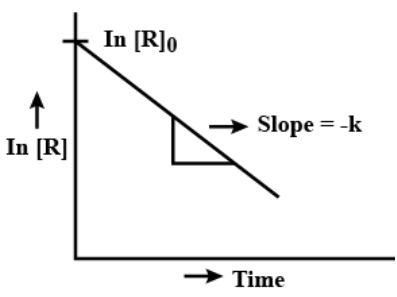
First Order: t 1/2 = 0.693/k
Example: Decomposition of H₂O₂.
Second Order Reaction
A + A → products (two molecules of the same reactant)
A + B → products (one molecule of two different reactants)
integrated rate expressions for both types:
A + A → products: The rate law is given by:
Rate = k[A] 2
Starting with:
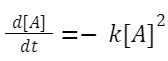
Integrating this expression from initial concentration
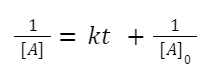
A + B→ products:
If the initial concentrations of A and B are not the same, then the math becomes a bit more involved. However, if the initial concentrations are equal ([A] 0 = [B] 0 ), the math simplifies:
The rate law is: rate = k[A][B]
If [A] 0 =[B] 0 , then the decrease in concentration of A and B will be the same at any given time, so [A]=[B].
The rate becomes: rate = k[A] 2
This will yield the same integrated rate expression as in the A + A → products case:
![rate = k[A]2](https://static.pw.live/5eb393ee95fab7468a79d189/GLOBAL_CMS_BLOGS/0688543f-2105-4a71-8643-c02337f3c16a.png)
If the initial concentrations are not equal, then the integrated rate expression needs to be derived taking into account the difference in concentrations, which is more complex.
Second Order: t 1/2 = k[A] 0
Graph:
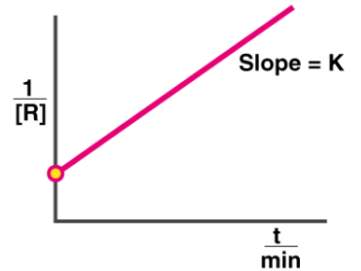
Example: Reaction between two ions to form a precipitate.
(Third-order reactions are rare and typically complex; we'll skip the equation for brevity.)
Temperature Dependence of Rate of Reaction
Also Check – Electronegativity Formula
Arrhenius Equation
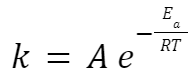
where: k is the rate constant.
A is the pre-exponential factor or frequency factor.
Ea is the activation energy.
R is the universal gas constant.
T is the temperature in Kelvin.
Collision Theory:
Molecules must collide. The colliding molecules must have energy greater than or equal to the activation energy Ea. The molecules should have proper orientation at the time of collision.
The rate is given by:
Rate α [A] [B] Z
Where: Z is the total number of collisions per second.
Collision Frequency
The number of collisions that occur in a given period of time per unit volume.
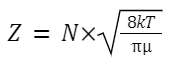
where: N is the number of molecules in the given volume.
k is the Boltzmann constant.
T is the absolute temperature.
μ is the reduced mass of the colliding particles,
given by:
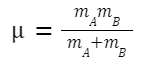
where m A and m B are the masses of molecules A and B, respectively.
Rate of Reaction based on Collision Theory:
Rate = Z AB × f× P
Where: Z AB is the collision frequency, which is the number of collisions happening per second between reactants A and B.
f is the fraction of collisions with energy ≥ Ea.
P is the probability that the collisions have the correct orientation.
Also Check – Percent by Weight Formula
Orientation Factor (P)
Not every collision leads to a reaction because molecules must collide in the correct orientation. This factor takes values between 0 and 1, representing the fraction of collisions that have the proper orientation for a reaction to occur. Considering the factors above, the effective rate of a reaction can be represented as:
Rate = PZ[A][B]exp(-E a /RT )
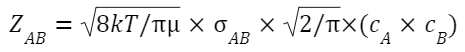
Where:
σ AB is the collision cross-sectional area for A and B.
cˉ is the average relative speed of A and B.
It can be given by the Boltzmann factor:
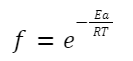
f is the fraction of collisions that have energy greater than or equal to the activation energy, E a.
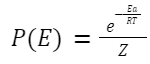
Where: Ea is the activation energy.
k is the Boltzmann constant.
T is the temperature in Kelvin.
P is the steric factor, representing the fraction of collisions with the correct orientation for reaction. This factor accounts for the fact that not all collisions, even if energetically favorable, lead to reaction. Its value is between 0 and 1.
Z is the partition function.
Chemical Kinetics Formula FAQs
Q1. How is reaction rate defined?
Q2. What is the order of a reaction?
Q3. What determines the rate of a first-order reaction?
Q4. What is the half-life of a reaction?
Q5. Is the half-life of a first-order reaction dependent on the initial concentration?










