
Density of Water
Sep 26, 2022, 16:45 IST
Water is an odorless, tasteless, transparent, and colorless chemical substance that is the main component of oceans, lakes, streams, and the fluids of most living organisms on this planet. Density is the mass per unit volume of a substance.
What is Density of Water
The Material density is defined as its weight per unit volume. It is a measure of how tightly the mass is packed together. The density of a substance or material can be defined as the relationship between the volume it occupies and the mass of a substance.
Density of Water At Room Temperature
A density of water is defined similarly to other substances. At room temperature (~200C), its value is 998.2 kg/m 3 . The density of water at 250 Celsius is 997 kg/m 3 . At room temperature, water remains in a liquid state. The density of distilled water is same. Seawater has salt and minerals that make it denser than normal water. At sea level, the density is about 1027 kg/m 3 .
Effect of Pressure on Density
Density increases as pressure increases and decreases as pressure decreases. As the pressure increases, the molecules of the substance are brought closer together, resulting in a higher density. On the other hand, when the pressure decreases, the molecules move apart. Due to this, the density decreases. This also happens with water.
Factors Affecting Water Density
Many factors can affect the density of a substance. Some of the factors that affect the density of water are given in the points below.
- The density of water is approximately 1 g/cm3 (1 g/cm 3 ).
- It is temperature-dependent, but this relationship is said to be non-linear and unimodal rather than monotonic.
- As it cools from room temperature, liquid water tends to become more and more dense, like other substances, but at about 4 °C pure water reaches its maximum density.
- As it cools further, it tends to expand and become less dense. This type of unusual negative thermal expansion is related to strong intermolecular forces, dependent on orientation or interactions, and is observed in the form of molten silica.
Density vs Temperature
Water does not have absolute density as its density varies with temperature. Water has a higher density in the liquid state than the solid. Check out the Density Vs Temperature graph mentioned below to understand how density changes with temperature.
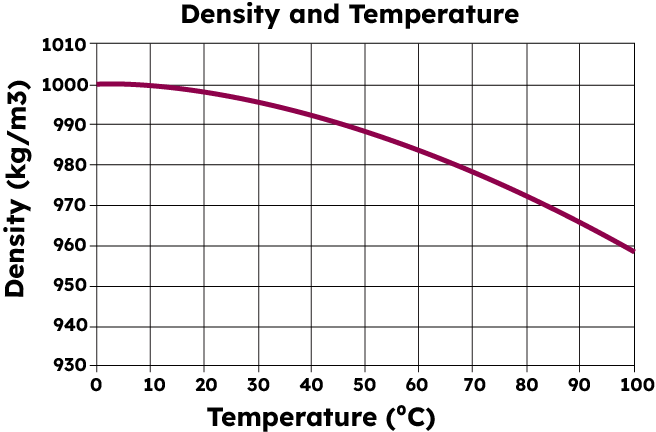
Density of Water at Various Temperature Scales
| Temperature | Density in kg/m 3 |
| 100 °C | 958.4 |
| 80 °C | 971.8 |
| 60 °C | 983.2 |
| 40 °C | 992.2 |
| 30 °C | 995.65 |
| 25 °C | 997.04 |
| 22 °C | 997.77 |
| 20 °C | 998.2 |
| 15 °C | 999.1 |
| 10 °C | 999.70 |
| 4 °C | 998.97 |
| 0 °C | 999.83 |
| -10 °C | 998.12 |
| -20 °C | 993.547 |
| -30 °C | 983.854 |
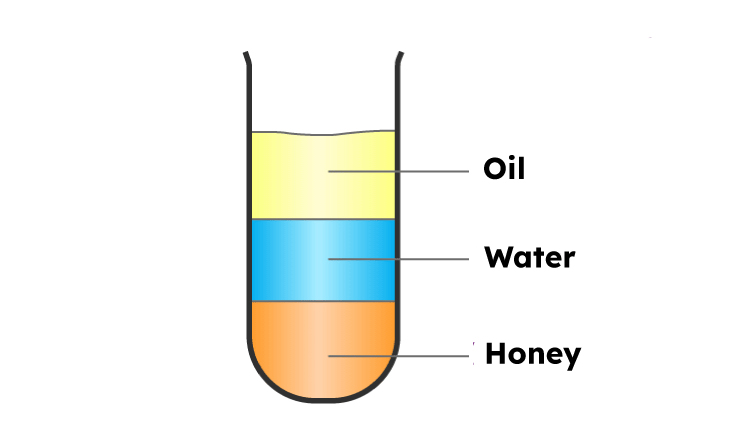
Density of Water Experiment
To know more about the density of water, let's do a little experiment. We will need a tall glass mug, honey, water, coconut oil, and food coloring.
Step 1: Pour a one-quarter cup of honey,
Step 2: Gently pour a one-quarter cup of dyed water over the surface of the honey.
Step 3: Pour a one-quarter cup of coconut oil over the colored water.
Now you'll notice that different substances have different densities, which means that for the same volume, different substances weigh differently because heavier substances tend to settle to the bottom, like honey, and lighter material, like oil, tends to float on top.
Density of several liquids with different specific gravity
| Material | Density (gram/cm 3 ) |
| Rubbing Alcohol | 0.79 |
| Lamp Oil | 0.8 |
| Baby Oil | 0.83 |
| Water | 1.0 |
| Milk | 1.03 |
| Liquid Soap | 1.06 |
| Corn Syrup | 1.33 |
| Maple Syrup | 1.37 |
| Honey | 1.42 |
Frequently Asked Questions (FAQs)
Q1. Is water density 997 or 1000?
Ans. Water has a density at 250 Celsius of 997 kg/m 3 . The density is known as the mass/volume. Pure water has a density of 1000kg/m 3 . or 1g/cm 3 .
Q2. What is density of freshwater?
Ans. 1 g/cm 3 at 4o C is the density of freshwater, but the addition of salts and other dissolved substances increases surface seawater density to between 1.02 and 1.03 g/cm 3 . The density of seawater can be increased by reducing its temperature, increasing its salinity , or increasing the pressure.
Q3. How to Calculate the density of Water Formula?
Ans. Density is usually denoted using the symbol ρ , so the formula for density is: ρ = m / V .
Q4. What is the maximum density of water?
Ans. The maximum density of water is around 4° Celsius. The density of ice is less than liquid water, thus, it floats. Upon freezing, the density of ice decreases by about 9%.
Q5. Does ice floats on water?
Ans. Ice is actually about 9% less denser than water. The water is heavier, so it displaces the lighter ice, causing the ice to float to the top.









