
Electrochemical Cell
Nov 10, 2022, 16:45 IST
The electrochemical cell is a device that is capable of producing electrical energy from the chemical reactions or by using electrical energy to cause chemical reactions.
| Table of Content |
What is Electrochemical Cell?
The electrochemical cell is a device that can produce electrical energy from chemical reactions occurring within it or also use the electrical energy supplied to it to facilitate the chemical reactions within it. These devices are capable of converting the chemical energy into the electrical energy or vice versa. A simple example of an electrochemical cell is the standard 1.5 volt cell used to power many electrical appliances such as television remote controls and clocks.
Such cells capable of generating electric current from the chemical reactions taking place in them are called galvanic cells or voltaic cells. Alternatively, cells that cause chemical reactions in them when the electric current is passed through them are known as electrolytic cells.
A diagram detailing the various parts of an electrochemical cell is shown below.
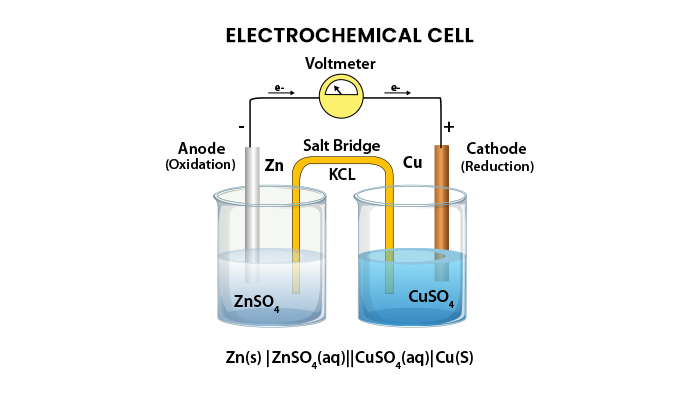
Electrochemical cells consist of cathode and anode. The key features of the cathode and anode are shown below-
| Cathode | Anode |
| Denoted by the positive sign since the electrons are consumed here | Denoted by the negative sign since the electrons are liberated here |
| A reduction reaction usually occurs in the cathode of the electrochemical cell | An oxidation reaction usually occurs here |
| Electrons move into the cathode | Electrons move out of the anode |
Half-Cells and Cell Potential
Electrochemical cells consist of two half-cells, each consisting of an electrode that is immersed in the electrolyte. The same electrolyte can also be used for both the half cells. These half-cells are connected by a salt bridge, which provides a platform for ionic contact between them without mixing with each other. An example of a salt bridge is filter paper that is immersed in a solution of potassium nitrate or sodium chloride.
One of the half cells of an electrochemical cell loses electrons due to an oxidation, and the other gains electrons in the process of reduction. It can be note that there is an equilibrium reaction in both the half cells, and when the equilibrium is reached, the net voltage, it becomes 0, and then the cell stops producing the electricity.
The tendency of an electrode in contact with the electrolyte to lose or gain an electrons is determined by its electrode potential. The values of the potentials can be used to predict the overall potential of the cell. In general, electrode potentials are measured using a standard hydrogen electrode as a reference electrode (electrode of known potential).
Primary and Secondary Cells
The primary cells are generally use-and-though galvanic cells. An electrochemical reactions take place in these cells are irreversible in the nature. Thus, the reactants are consumed to produce electricity, and the cell stops producing electric current once the reactants are entirely exhausted.
The secondary cells (also called rechargeable batteries) are the electrochemical cells in which the cell has reversible reaction, i.e., the cell can function as an galvanic cell as well as an electrolytic cell.
Most primary batteries (multiple cells connected in series, parallel, or a combination of both) are considered wasteful and environmentally harmful devices. This is because they need about 50 times the energy they contain in their manufacturing process. They also have many toxic metals and are considered hazardous waste.
Types of Electrochemical Cells
There are two primary types of electrochemical cells are-
- Galvanic cells (also known as Voltaic cells)
- Electrolytic cells
The differences between Galvanic cells and electrolytic cells are mentioned below-
| Galvanic Cell / Voltaic Cell | Electrolytic Cell |
| Chemical energy is converted into the electrical energy in these electrochemical cells. | Electrical energy is converted into the chemical energy in these cells. |
| The redox reactions take place in these cells are spontaneous in the nature. | Energy input is required for the redox reactions to proceed in these cells, i.e., the reactions are non-spontaneous. |
| These electrochemical cells, experience a negatively charged anode, and the cathode is positively charged. | These electrochemical cells experience a positively charged anode and the negatively charged cathode. |
| Electrons originate from the species that undergo oxidation. | Electrons originate from an external source (such as a battery). |
Applications of Electrochemical Cells
- An electrolytic cell are also used in the electrorefining of many non-ferrous metals. They are also used in the electrolytic recovery of these metals.
- High-purity lead, zinc, aluminum, and copper production involves electrolytic cells.
- Sodium metal can be extracted from the molten sodium chloride by just placing it in the electrolytic cell and then passing an electric current through it.
- Many commercially essential batteries (such as the lead acid battery) are made up of galvanic cells.
- Fuel cells are an essential class of electrochemical cells that serve as a source of clean energy in several remote locations.
Frequently Asked Question (FAQs)
Q1. What factors affect electrochemical cells?
Ans. According to Gibbs Free energy, concentration, temperature, and gas pressure affect the electrochemical cells. The Gibbs free energy measures how far a system is from equilibrium. It, therefore, determines an electrochemical cell's voltage (driving force).
Q2. Why electrochemical cell stops working?
Ans. An electrochemical cell stops working after some time because the electrode potential of both electrodes becomes equal.
Q3. What is the popular name for electrochemical cell?
Ans. Galvanic cell or voltaic cell
Q4. What is the maximum voltage of a cell?
Ans. The maximum voltage of a cell is 4.2v and the average voltage is 3.7V.
Q5. What are the disadvantages of using electrochemical cells?
Ans. Few are the disadvantegs of using electrochemical cells- cannot be recycled, can leak (weak acid electrolyte reacts with zinc), short shelf–life, unstable voltage and current (as battery 'runs down') and low power.









