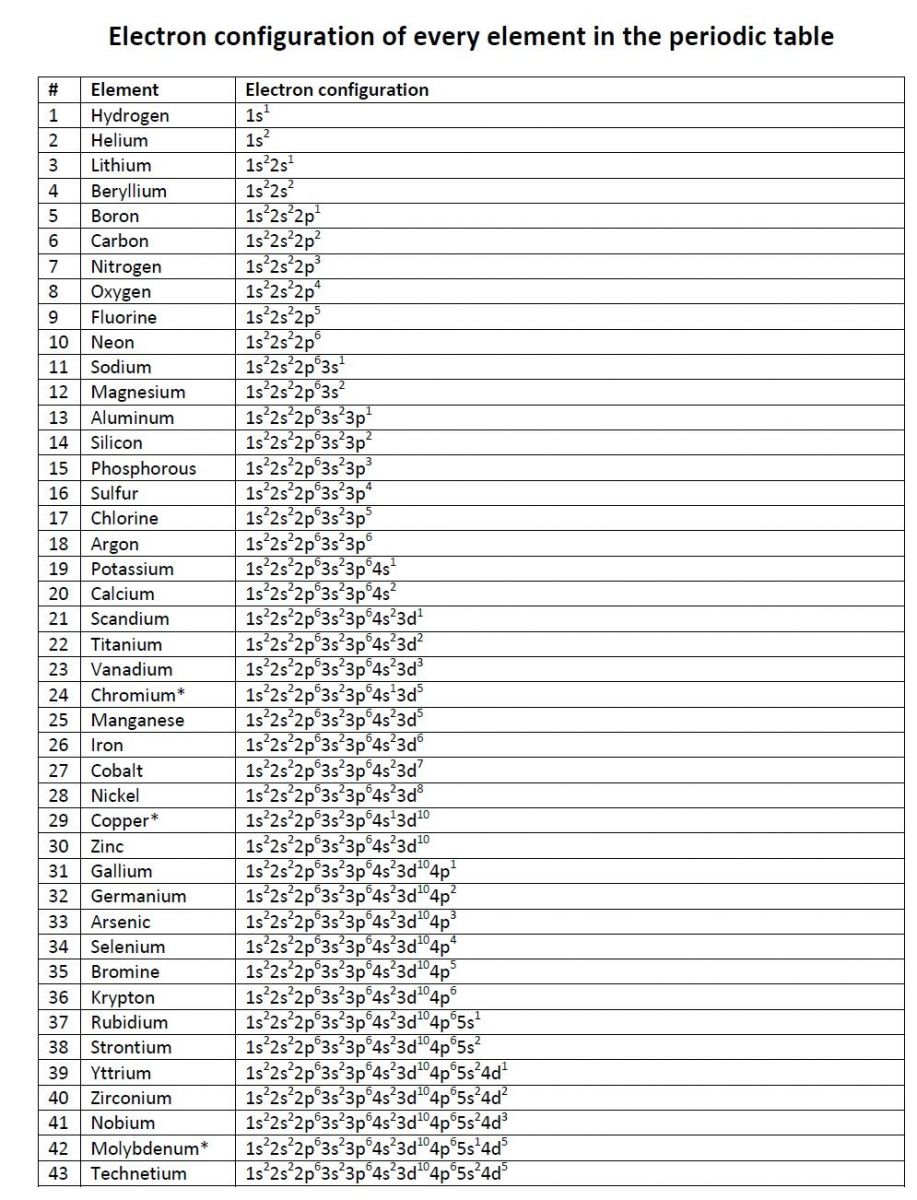
Electronic Configuration of Elements
Aug 26, 2022, 16:45 IST
What is Electronic configuration
The electron configuration of an atom is the particular distribution of electrons among available shells. It is described by a notation that lists the subshell symbols, one after another. Each symbol has a superscript on the right giving the number of electrons in that subshell. For example, a configuration of the lithium atom (atomic number 3) with two electrons in the 1s subshell and one electron in the 2s subshell is written 1s 2 2s 1 . The notation for electron configuration gives the number of electrons in each subshell.
Rules to write Electronic configuration of elements
The atom is built up by filling electrons in various orbitals according to the following rules.
Aufbau Principle: This principle states that the electrons are added one by one to the various orbitals in order of their increasing energy starting with the orbital of lowest energy. The increasing order of energy of various orbital is
1s,2s,2p,3s,3p,4s,3d,4p,5s,4d,5p,6s,4f,5d,6p,5f,6d,7p……………………
How to remember such a big sequence? To make it simple we are giving you the method to write the increasing order of the orbitals. Starting from the top, the direction of the arrows gives the order of filling of orbitals.
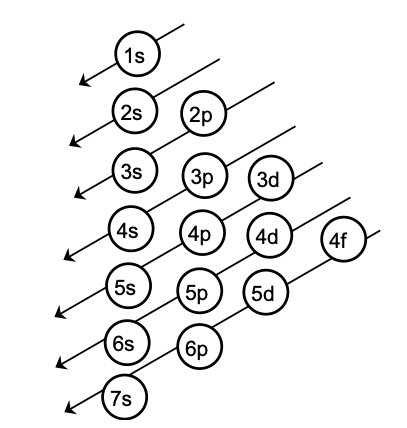
Alternatively, the order of increasing energies of the various orbitals can be calculated on the basis of (n+ l ) rule.
The energy of an orbital depends upon the sum of values of the principal quantum number (n) and the azimuthal quantum number ( l ). This is called (n+ l ) rule. According to this rule,
In neutral isolated atom, the lower the value of (n+ l) for an orbital, lower is its energy. However, if the two different types of orbitals have the same value of (n+ l), the orbitals with lower value of n has lower energy.
Illustration of (n + l) rule
Type of orbitals Value of n Values of l Values of (n+ l) Relative energy
1s 1 0 1+0=1 Lowest energy
2s 2 0 2+0=2 Higher energy than 1s
2p 2 1 2+1=3 2p orbital (n=2) have lower energy than 3s orbital (n=3)
(n +l) rule is applicable for multi electronic systems only. For uni-electronic system like H, order of energy of orbitals is not “significantly influenced” by
![]() .
Now can you write the order of energy of orbitals for uni-electronic system?
.
Now can you write the order of energy of orbitals for uni-electronic system?
Important principle use while write Electronic configuration of elements
Pauli’s Exclusion principle
According to this principle, an orbital can contain a maximum number of two electrons and these two electrons must be of opposite spin.
Two electrons in an orbital can be represented by ||
Hund’s rule of maximum multiplicity
This rule deals with the filling of electrons in the equal energy (degenerate) orbitals of the same sub shell (p,d and f). According to this rule,
Electron pairing in p,d and f orbitals cannot occur until each orbital of a given subshell contains one electron each or is singly occupied & that too with the same spin.
This is due to the fact that electrons being identical in charge, repel each other when present in the same orbital. This repulsion can, however, be minimized if two electrons move as far apart as possible by occupying different degenerate orbitals. All the electrons in a degenerate set of orbitals will have same spin.
Multiplicity is given by 2|S| + 1. Can you now comment why the rule is called Hund’s rule of maximum multiplicity.
How to write Electronic configuration of elements
Electronic configuration is the distribution of electrons into different shells, subshells and orbitals of an atom.
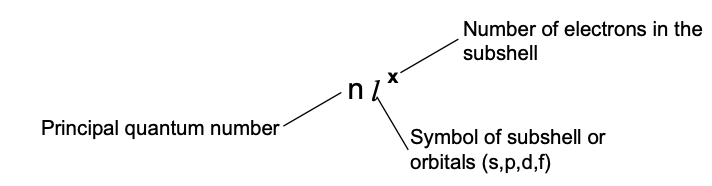
Orbital can be represented by a box and an electron with its direction of spin by arrow. To write the electronic configuration, we need to know (i) the atomic number (ii) the order in which orbitals are to be filled (iii) maximum number of electrons in a shell, sub–shell or orbital.
(A) Each orbital can accommodate two electrons
(B) The number of electrons to be accommodated in a subshell is 2 multiply by number of degenerate orbitals.
Subshell Maximum number of electrons
s 2
p 6
d 10
f 14
(C) The maximum number of electron in each shell (K,L,M,N…) is given by 2n2. Where n is the principal quantum number.
(D)The maximum number of orbitals in a shell is given by n2 where n is the principal quantum number.
Exceptional Configurations (Extra stability of half-filled and fully-filled sub shell)
The electronic configuration of most of the atoms follow the Aufbau’s rule. However, in certain elements such a Cr, Cu etc. electron fills in 3d in preference to 4s provided the subshell become either half-filled or fully filled.
24
Cr
![]() [Ar] 3d
5
, 4s
1
and not [Ar] 3d
4
, 4s
2
[Ar] 3d
5
, 4s
1
and not [Ar] 3d
4
, 4s
2
29
Cu
![]() [Ar] 3d
10
4s
1
and not [Ar] 3d
9
, 4s
2
[Ar] 3d
10
4s
1
and not [Ar] 3d
9
, 4s
2
It has been found that there is extra stability associated with these electronic configurations. This stabilization is due to the following two factors
1.Symmetrical distribution of electron:
It is well known that symmetry leads to stability. The completely filled or half-filled subshells have symmetrical distribution of electrons in them and are therefore more stable. This effect is more dominant in d and f-orbitals.
This means three or six electrons in p-subshell, 5 or 10 electrons in d-subshell, and 7 or 14 electrons in f-subshell forms a stable arrangement.
2.Exchange Energy:
This stabilizing effect arises whenever two or more electrons with the same spin are present in the degenerate orbitals of a subshell. These electrons tend to exchange their positions and the energy released due to this exchange is called exchange energy. The number of exchanges that can take place is maximum when the subshell is either half-filled or full filled. As a result the exchange energy is maximum and so is the stability.
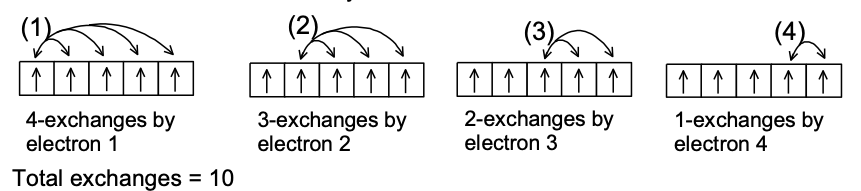
If n is the number of electron with parallel spins then can you calculate total number of possible exchanges?
Electronic configuration of ions
Note that while filling electrons in various orbitals they are filled according to the three laws – Aufbau, Pauli and Hund’s. For removing electrons to form cations, electrons are removed from outermost shell as they are bound to the nucleus by lesser forces of attraction because of shielding effect.
For example for iron,
26
Fe
![]() 1s
2
, 2s
2
2p
6
, 3s
2
3p
6
3d
6
, 4s
2
& the configuration of ions would be
1s
2
, 2s
2
2p
6
, 3s
2
3p
6
3d
6
, 4s
2
& the configuration of ions would be
Fe
2+
![]() 1s
2
, 2s
2
2p
6
, 3s
2
3p
6
3d
6
& Fe
3+
1s
2
, 2s
2
2p
6
, 3s
2
3p
6
3d
6
& Fe
3+
![]() 1s
2
, 2s
2
2p
6
, 3s
2
3p
6
3d
5
1s
2
, 2s
2
2p
6
, 3s
2
3p
6
3d
5
Similarly for copper
29
Cu
![]() 1s
2
, 2s
2
2p
6
, 3s
2
3p
6
3d
10
, 4s
1
& for its ions
1s
2
, 2s
2
2p
6
, 3s
2
3p
6
3d
10
, 4s
1
& for its ions
Cu
+
![]() 1s
2
, 2s
2
2p
6
, 3s
2
3p
6
3d
10
& Cu
2+
1s
2
, 2s
2
2p
6
, 3s
2
3p
6
3d
10
& Cu
2+
![]() 1s
2
, 2s
2
2p
6
, 3s
2
3p
6
3d
9
1s
2
, 2s
2
2p
6
, 3s
2
3p
6
3d
9
The anions are formed by adding electrons to the vacant orbital of lowest energy [follow (n +l) rule]
For example
9
F
![]() 1s
2
, 2s
2
2p
5
& that of its ion F
-
1s
2
, 2s
2
2p
5
& that of its ion F
-
![]() 1s
2
, 2s
2
2p
6
1s
2
, 2s
2
2p
6
Similarly for Chlorine
17
Cl
![]() 1s
2
, 2s
2
2p
6
, 3s
2
3p
5
& that of its ion Cl
-
1s
2
, 2s
2
2p
6
, 3s
2
3p
5
& that of its ion Cl
-
![]() 1s
2
, 2s
2
2p
6
, 3s
2
3p
6
1s
2
, 2s
2
2p
6
, 3s
2
3p
6
Some Exceptional electronic configuration :
24 Cr: 1s 2 , 2s 2 2p 6 , 3s 2 3p 6 3d 5 , 4s 1
47 Ag : 1s 2 , 2s 2 2p 6 , 3s 2 3p 6 3d 10 , 4s 2 4p 6 5s 1
29 Cu: 1s 2 , 2s 2 2p 6 , 3s 2 3p 6 3d 10 , 4s 1
42 Mo: 1s 2 , 2s 2 2p 6 , 3s 2 3p 6 3d 10 , 4s 2 4p 6 4d 5 , 4s 1
List of Electronic configuration of elements