
Molecular Chromic Acid - H 2 CrO 4 is similar to sulphuric acid (H 2 CrO 4 ) because both are strong acids, but only the first proton is easily lost.
Chromic acid, H 2 Cr 2 O 7 , is the protonated form of the dichromate ion (Cr 2 O7 – ). It also forms when chromium trioxide (CrO 3 ) is added to molecular chromic acid.
Chromic Acid
In addition to being the lightest element, hydrogen has the symbol H and has an atomic number of 1 and an electron configuration of 1s1. It is colorless, odorless, tasteless, non-toxic, and highly combustible. As an extremely flammable gas, it burns air and oxygen to produce water. It is used in the production of nitrogenous fertilizers, rocket fuel, and hydrochloric acid, among other uses.
The chemical element chrome is represented by the symbol Cr. It is a steely gray, lustrous, hard, and brittle transition metal with an atomic number of 24 and an electronic configuration of [Ar]3d5 4s1. It does not occur as a free element in nature but is found in ores. Chromite is the main ore of chromium.
A colorless, odorless and tasteless gas essential to living organisms, oxygen is a chemical element with the symbol O and an atomic number of 8. Water, rocks, minerals, and numerous organic compounds contain this reactive element. It is the most abundant element on the planet. As a gas, it supports life and is extremely flammable.
Properties of Chromic Acid H 2 CrO 4
Chromic acid has the following properties.
- In organic chemistry, chromic (VI) acid is a commonly used reagent known for its oxidizing properties. It is used to oxidize alcohols to carboxylic acids and ketones.
- Chromic (VI) Acid is not combustible, but it accelerates combustion of combustible materials.
- H2CrO4 is its chemical formula or H 2 Cr 2 O 7
- The crystals are dark red in color.
- Chromic (VI) acid has a density of 1.201 g cm−3
- 197 °C (387 °F; 470 K) is its melting point, and 250 °C (482 °F; 523 K) is its boiling point.
- A solubility of 169 g/100 mL makes it readily soluble in water.
- Acidity (pKa) ranges from -0.8 to 1.6.
- Chromate and dichromate are the conjugate bases of this acid.
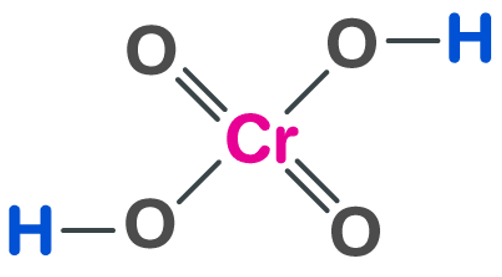
Structure of Chromic Acid (H 2 CrO 4 )
A strong oxidizing agent, chromic acid begins with the letter H. When we look at the name of the chemical, there is no prefix. All acids contain hydrogen. In this structure, hydrogen bonds with the metal chromate. During the formation of chromic acid, four oxygen atoms are bonded to chromium. Two of them have double bonds and two have single bonds, and each oxygen atom is bonded to a hydrogen atom.
Also Check – Value of Gas Constant Formula
Preparation of Chromic Acid
Molecular acid is formed when chromic trioxide is added to water.
CrO 3 + H 2 O → H 2 CrO 4
The reverse reaction occurs when molecular acid is dehydrated. This is often what happens when concentrated vitriol is added to a dichromate solution. The colour changes from orange to red (chromic acid). Chromium trioxide crystals precipitate from the mixture, but their colour does not change further.
LMCT transitions result in the colours. Chromium trioxide is anhydride of molecular acid. It is a Lewis acid and can react with Lewis bases. Pyridine reacts in non-aqueous media. An adduct of chromium trioxide and pyridine, Collins reagent can be used in a wide variety of oxidations. During its oxidation of alcohols in aqueous solutions, it (H2CrO4) makes chromic ester. During this reaction, the oxygen atom bridges the carbon and chromium atoms.
Also Read : Acetone Formula
Uses of Chromic Acid (H 2 CrO 4 )
- The chromium plating process uses chromic acid as an intermediate.
- Ceramic glazes and colored glass contain it.
- To clean laboratory glassware, chromosulfuric acid or sulfochromic mixture is used.
- In instrument repair, it is used to brighten raw brass.
- It was used in hair dye in 1940.
The fully protonated state of the dichromate ion is known as dichromic acid, or H2Cr2O7. Combining chromium trioxide with molecular chromic acid can also form this compound. Introducing dichromic acid to an aldehyde or ketone exhibits the same reaction behavior in organic chemistry. This substance can effectively oxidize primary alcohols to aldehydes and secondary alcohols to ketones. However, tertiary alcohols and ketones remain unaffected by its oxidizing properties. The color of chromic acid will shift from orange to brownish green during the oxidation process.
Chrome acid has been shown to oxidize many forms of organic compounds, and many variants have been developed for it. In aqueous sulfuric acid and acetone, chromic acid is known as the Jones reagent because it oxidizes primary and secondary alcohols into carboxylic acids and ketones, but rarely affects unsaturated bonds. Inorganic chemistry uses chromic acid to test for chloride ions. Cromyl chloride is formed from chromic acid.
The mixture of chromium trioxide and pyridinium chloride produces pyridinium chlorochromate. This reagent converts into the corresponding aldehydes (R-CHO).
Also Read : Acetone Formula
Health Hazards
Because hexavalent chromium compounds like chromium trioxide, chromates, chromic acids, and chlorochromate are toxic and carcinogenic, chromic acid oxidation is only used in aerospace.
A dilute solution of sodium thiosulfate is used in case of any burns caused by these acids since they are strong oxidizers and can react violently if mixed with some easily oxidizable organic substances.
Chromic Acid Formula FAQs
Q1. What is the chemical formula of chromic acid?
Q2. Is chromic acid a strong or weak acid?
Q3. What is the primary use of chromic acid?
Q4. Is chromic acid highly corrosive and toxic?
Q5. Why has the use of chromic acid declined in recent years?










