
The Condensed Structural Formula is basically a system for writing organic molecules in a line of text. We write them in shorthand notation that contains more detail than the molecular formula. However, it is important to note that the condensed structural formula is smaller when compared to the normal structural formula.
Condensed Structural Formula
It is written down simply in one line listing the atoms in the order in which they occupy the molecule. Furthermore, it shows the functional groups that are present in the molecule.
By using amine-NH2, alcohol -OH and parenthesis, we are showing that polyatomic groups are part of the same chain. For example, methane is CH 4 and propanol is CH 3 (CH 2 ) 2 OH.
Thus, in condensed structural formula, every carbon atom is signified individually, and subsequent carbon atoms are positioned to the atoms that bond to that specific carbon atom. This is similar to a chemical reaction in which the first carbon atom contains 3 hydrogen atoms, the second carbon atom contains 2 hydrogen atoms, the third carbon atom contains 3 hydrogen atoms, and the fourth carbon atom contains 3 hydrogen atoms.
Another more condensed method of writing structural formulas is when there are two CH 2 groups in the middle of the molecule, followed by (CH 2 ) 2 .
Also Check – Lithium Oxide Formula
Formula
The three types of formulas in organic chemistry should be distinguished in order to avoid any confusion.
A molecular formula (the number of atoms in each element) is C5H12O.
Condensed structural formula (shows all atoms but acknowledges vertical bonds and polyatomic groups in parenthesis)
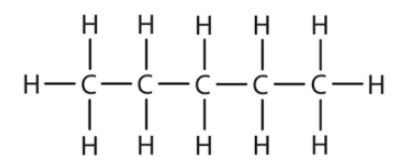
(Illustrates all the bonds in the structural formula)
The condensed structural formula uses lines between bonded atoms as well. However, it is a simpler and shorter way to draw the line-bond structural formula as it ignores the carbon and hydrogen bonds. It is apparent when comparing the condensed structural formula to the line-bond structural formula that this is the case.
Also Check – Periodic Acid Formula
Uses
It is particularly beneficial to show the functional groups of a molecule. Essentially, functional groups are responsible for determining how each molecule behaves chemically and physically. A chemist can use this information to estimate the behavior of compounds based on the number and type of functionality of these groups.
Additionally, the condensed structural formula also displays the geometry of some molecules. Furthermore, it is a key part of recognizing the properties of chemical compounds.
Condensed Structural Formula FAQs
Q1. What is a condensed structural formula?
Q2. How does a condensed structural formula differ from a full structural formula?
Q3. Can you provide an example of a condensed structural formula?
Q4. What are the advantages of using condensed structural formulas?
Q5. Are condensed structural formulas unique for each compound?










