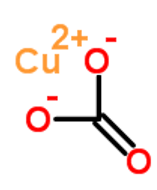
In addition to this, it is an ionic solid compound containing copper (II) cations Cu 2+ and carbonate anions CO 2 . Since it is difficult to prepare, the Copper II Carbonate Formula can be hard to find.
Most commonly, copper carbonate or cupric carbonate is a basic copper carbonate like Cu 2 (OH) 2 CO 3 . It is found in nature as the mineral malachite or Cu 3 (OH) 2 (CO 3 ) 2 , also known as azurite. This means the qualifier neutral can be used instead of basic since CuCO 3 is a particular case.
Structural Formula of Copper II Carbonate
As shown in the figure below, Copper II Carbonate reacts immediately with water and air and is a tedious process to prepare practically.
Preparation of Copper II Carbonate
A variety of experiments and reactions, including the following obtained CuCO 3 :
- In ambient conditions, solutions of Copper II Sulfate CuSO 4 and sodium carbonate Na 2 CO 3 are mixed. This results in the formation of a basic carbonate and CO 2 , due to the high reactivity of the Cu 2+ ion toward its hydroxide anion.
- Afterwards, the carbonate is thermally decomposed at atmospheric pressure to yield Copper II oxide CuO instead of the carbonate.
- The synthesis of copper carbonate was claimed by W. F. T. Pistorius in 1960 by heating the basic copper carbonate at 180 degrees C in 450 atm of carbon dioxide and 50 atm of water for 36 hours. Malachite Cu 2 CO 3 (OH) 2 was obtained in bulk, but a small quantity of rhombohedral substance was also obtained, which was attributed to CuCO 3 . Since this synthesis was not very reliable, it was discontinued.
- Hartmut Ehrhardt and others reported the reliable synthesis of true Copper II Carbonate in 1973. During the decomposition of silver oxalate Ag 2 C 2 O 4 at 500 °C and 2 GPa (20,000 atm), basic copper carbonate was heated in carbon dioxide, which was produced by the decomposition of basic copper carbonate. Monoclinic structure was reported for the obtained compound.
Also Check – Aluminium Acetate Formula
Physical Properties of Copper II Carbonate
Copper II Carbonate has the following physical properties:
- Compounds of this type are solids.
- Crystals with monoclinic faces
- At room temperature, in a sealed, dry place.
Also Check – Chemical Bonding Formula
Chemical Properties of Copper II Carbonate
Copper II Carbonate has the following chemical properties:
- When heated in the air, it breaks down into copper oxide, water, and carbon dioxide.
- A copper salt is also formed when it is dissolved in acid.
- A copper complex is formed in aqueous solutions of cyanide, ammonium salt, and alkali metal carbonate.
- Boiling or heating in an alkaline solution generates brown oxide.
- Copper sulfide is very unstable under hydrogen sulfide atmospheres and can react with hydrogen sulfide to form copper oxide.
- It slowly turns into a green malachite composition after being exposed to the air for a long time.
- CuCO 3 remains stable for months in dry air but decomposes slowly into CuO and CO 2 if P CO2 is less than 0.11 atm.
- CuCO 3 is constant only for p CO2 above 4.57 atmospheres and a pH between 4 and 8 in water or moist air at 25°C.
- At low partial pressures, it forms a basic carbonate - (azurite, Cu 3 (CO 3 ) 2 (OH) 2 ).
Also Check – Bleaching Powder Formula
Uses of Copper II Carbonate
- A variety of applications are possible with copper carbonate. It is frequently used to treat wood with compounds.
- It is an active ingredient in animal feeds and in high demand in animal fodder. Copper carbonate is combined with arsenic to make acetoarsenite, sometimes called Paris green.
- When turning copper into copper salts, copper carbonate is first treated with a powerful acid. Water and carbon dioxide gas are then introduced.
- Water, cupric acid, and carbon dioxide can be produced when vinegar (acetic acid) is combined with carbonate. It is also used for a variety of esthetical purposes. Jewelry is one of the most prominent applications.
- The distinct colors of each component make them perfect for use as colorants and pigments. Combined in their purest state, they create a refreshing mint green shade. Adding alkaline components will produce a subtle blue tint. These hues are highly effective for coloring various products, such as paints and varnishes.
- Many artists rely on copper carbonate, also known as verditer or mountain green, to achieve their desired shades in their paints. This versatile ingredient is also commonly used in fireworks and ceramic glazes as a popular pigment and colorant.
- Copper Carbonate is a byproduct of Copper Chromite catalyst production, which is commonly used in the industrial-scale hydrogenolysis of fatty methyl esters to form fatty alcohols. This catalyst also exhibits high hydrogenation activity for converting aldehydes and ketones to alcohols and nitro-compounds to primary amines.
- In order to control reaction rate and optimize synthesis in the reactor, some manufacturers utilize Copper Oxide Black instead of Copper Carbonate.
Copper II Carbonate Formula FAQs
Q1. What is the chemical formula of chromic acid?
Q2. Is chromic acid a strong or weak acid?
Q3. What is the primary use of chromic acid?
Q4. Is chromic acid highly corrosive and toxic?
Q5. Why has the use of chromic acid declined in recent years?