
science class 10th chapter-Periodic classification Formula & Important points
May 13, 2022, 16:45 IST
Class-10 for chapter-Periodic classification
Classification of Elements
The need to simplify and organize the study of elements and their large number of compounds led to the development of the periodic table. Henry Moseley in 1913 showed that atomic number was a more fundamental property of an element than its atomic mass. Therefore, atomic number or electron number was adopted as the basis of classification of elements.
Modern Periodic Law
Mendeleev's periodic law was thus modified to Modem periodic law which states that the properties of elements are a periodic function of their atomic number.
Salient Features of Modern Periodic Table
- When the elements are arranged in increasing order of their atomic numbers, the anomalies of Mendeleev's periodic table are removed. However, the position of hydrogen still remains anomalous. It can be placed either along with all metals of group 1 or along with halogens of group 17 of the Modern periodic table.
- In the Modern or Long form of the periodic table , elements are arranged in increasing order of their atomic numbers.
- The Modern periodic table is based upon electronic configuration of elements.
- The periodicity in properties of elements is due to periodicily in their outer electronic configurations.
- The numbers 2, 8, 8, 18, 18 and 32 after which the properties of elements get repeated are the magic numbers on which this classification is based.
- The Modern periodic table consists of 18 vertical columns called groups and 7 horizontal rows called periods.
- Each period starts with the filling of electrons in a new electronic shell and the elements in a period have consecutive atomic numbers
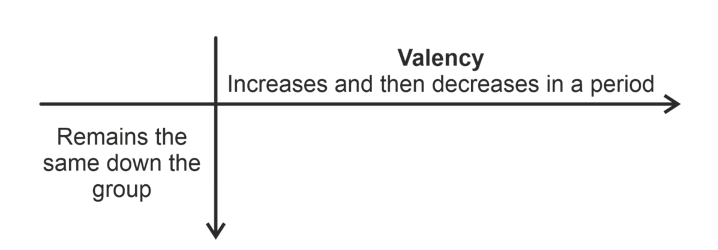
Valency
The valency of elements in a group is fixed but in a period first it increases from 1 to 4 and then decreases to zero.
Atomic size/Atomic Radii
The atomic size decreases across a period from left to right but increases down a group.
Do solve NCERT questions with the help of NCERT solutions for class 10 Science prepared by academic team of Physics Wallah.
Download Free Pdf sheet of Periodic classification it consist of Short notes and key points of Chemistry for class 10 from the link given below.









