
Important points and formula of chemistry for class 9 chapter-Matter in our surrounding
Jul 27, 2022, 16:45 IST
Everything in this universe is made up of matter. Matter is defined as anything which occupies space, possesses mass and the presence of which can be felt by any one or more of our five senses, i.e., sight, touch, smell, hearing and taste.
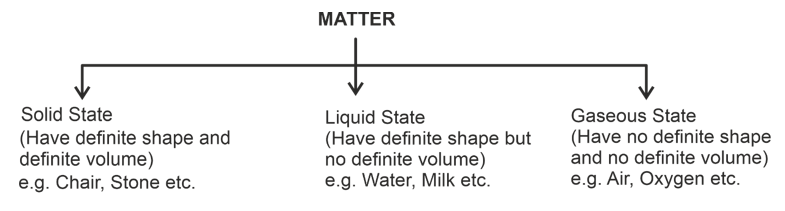
States of Matter
- Directly proportional to the product of the masses of the objects and
- Inversely proportional to the square of the distance between their centres
Matter around us exists in three states, i.e., solid, liquid and gas. These three states differ from one another dur to the difference in the size of spaces in between the constituent particles, their forces of attraction and kinetic energies.
Differences in the characteristics of states of matter (solids, liquids & gases)
| S.No. | Property | Solid | Liquid | Gas |
| 1. | Packing | The particles are most closely packed |
The particles are less closely
packed than solids. |
Particles are at sufficient distances
from each other. |
| 2. | Shape | Solids have definite shape. |
Liquids do not have definite shape.
They assume the shape of container. |
Gases do not have a definite shape.
They assume the shape of container. |
| 3. | Volume | Solid have definite volume. | Liquids have definite volume. |
Gases do not have definite volume.
They assume the volume of container. |
| 4. | Density | Solids have high density. |
Liquids have less density than
solids but more than gases. |
Gases have the least density. |
| 5. | Diffusion | Solids have no tendency to diffuse. | Liquids have a tendency to diffuse slowly. | Gases diffuse rapidly. |
| 6. | Rigidity | Rigid. | Fluid. | Fluid. |
| 7. | Compressibility | Negligible. | Very low. | High. |
| 8. | Inter-molecular forces of attraction | Maximum. | Less than solids. | Negligible. |
| 9. | Kinetic energy of molecules | Least. | More than solids. | Very high. |
Do solve NCERT Solution with the help of NCERT Solutions for class 9 Science prepared by academic team of Physics Wallah. For additional information related to the subject you can check the Science Formula section.
Download Free Pdf sheet of Important points & formula of chemistry for class 9 chapter-Matter in our surrounding from the link given below.









