
A neutron is a neutral subatomic particle. As we know, atoms are small and so are their interior entities. Neutrons are one such entity with no charge. The neutron, its rest mass, its mass, its relative charge, and its relative mass will be discussed in this topic.
Discovery of Mass of Neutron
In 1906, physicist Ernest Rutherford conducted an investigation into the composition of atoms. His findings revealed that the majority of an atom's mass and positive charge is centralized in its core.
Later, in 1932, James Chadwick also conducted an experiment involving bombarding beryllium with alpha particles. During this experiment, he observed a release of neutral radiation.
At the time, the only known type of neutral radiation was photons, but the energy emitted during Chadwick's experiment was significantly lower. As a result, he named these particles "neutrons."
Formation of Mass of Neutron
The formation of neutrons in the cosmos occurs through fusion in stars' cores. In one reaction, a high-energy cosmic ray proton collides with an atomic nucleus, resulting in the production of a neutron and a hydrogen nucleus (H). This can be expressed as proton + atomic nucleus → neutron + H. Conversely when a high-energy neutron collides with an atomic nucleus, it creates a proton, an electron (e), and an anti-neutrino (ν) in the opposite reaction: neutron + atomic nucleus → proton + e + anti-neutrino. As these particles are formed, they are left to move quickly on their own, making it challenging to capture them due to their immense kinetic energy.
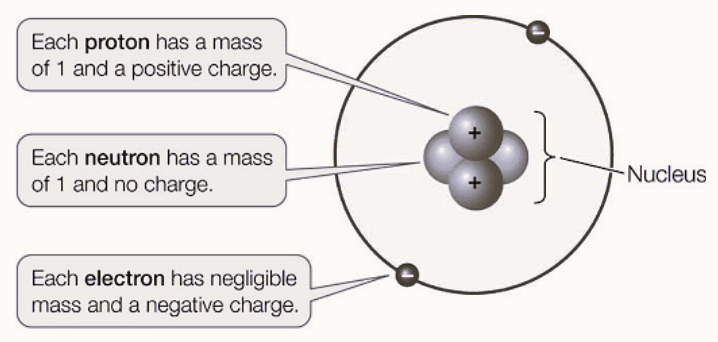
Neutron Mass
The neutron has a mass of approximately 1.00866491588 atomic mass units (u) or unified atomic mass units (amu). In kilograms, this is roughly equal to 1.675 x 10 -27 kg. Neutrons, along with protons, make up the nucleus of an atom and are collectively referred to as nucleons. They contribute significantly to the mass of an atom, as electrons, which orbit the nucleus, have much smaller masses compared to nucleons.
Also Read – Malic Acid Formula
Neutron Mass in Grams
Since 1kg = 103 grams
mn = 1.67493 × 10-27 × 103
mn = 1.67493 × 10-24 gm
Neutron Mass in atomic mass unit(amu)
Since, 1 kg = 6.0229552894949E + 26 amu
Therefore,
1.67493 × 10-27 kg = 1.67493 × 10-27× 6.0229552894949E + 26 amu
mn = 1.008664904(14) amu
Also Check – Iron (III) Hydroxide Formula
Mass of One Neutron
Mass of a free neutron = 1.67493 × 10-27 kg
Now we will change it to electron volts,
We know that, 1 eV = 1.6 × 10-19 J
and Speed of light, c = 3 × 108 m/s
Since, 1 kg = 5.6095883571872E + 35eV
Therefore,
1.67493 × 10-27 kg = (5.6095883571872E + 35eV )× 1.67493 × 10-27
mn = 939,565,413.3 eV
Also, in Mega electron Volts (MeV),
mn = 939.565346 MeV/C2
Relative Mass of Neutron
- Protons, electrons, and neutrons are the subatomic particles of atoms.
- Protons and neutrons reside in an atom's nucleus, which is at its center. 4Protons are as massive as neutrons; we can say approximately 99.86 %, and electrons are 0.054% heavier.
- The relative mass of each particle is measured in kilograms.
- The neutron has a relative mass of 1.
Also Read: Molar Volume Formula
Things to Remember About Mass of Neutron
- A neutron, being neutral, has no electrical charge.
- Its mass is nearly equal to that of a proton and it is one of three components that make up an atom.
- The mass of a neutron can be expressed in different units: 1.67493 × 10-24 gm or 1.008664904(14) amu or 1.67493 × 10-27 kg.
- Alternatively, the mass of a single neutron can also be described as 939,565,413.3 eV and its relative mass is simply 1.
- James Chadwick first introduced the concept of protons and neutrons comprising the nucleus upon his discovery of the neutral particle known as a neutron.
- Interestingly, the relative charge on a neutron is precisely zero.
Mass of Neutron FAQs
Q1. What is the mass of a proton and neutron?
Q2. What is the mass of a neutron in kilograms (KG)?
Q3. Is the neutron mass 1 or 0?
Q4. What is the mass of a proton?










