
Iron III Hydroxide Formula has the chemical formula Fe(OH)3 and comprises iron, hydrogen, and oxygen. The color of iron III hydroxide varies from dark brown to black depending on its hydration, crystal structure, and particle size. Iron III hydroxide is also known as ferric hydroxide.In humid conditions, iron(III) hydroxide is created due to rusting. Solid Fe³⁺ ions are produced from solid iron during this process, which involves the interaction of H⁺ and O₂. To accurately depict the various steps, net ionic equations must be formulated to ensure that both sides possess the same total charge. If state symbols are also included, a balanced net ionic equation for the transfiguration of metallic iron with hydrogen ions and oxygen molecules into Fe³⁺ ions can be given.
Characteristics of Iron III Hydroxide
| Name | Iron III Hydroxide |
| Also known as | Ferric acid |
| Appearance | Vivid dark orange crystals |
| Chemical Formula | Fe(OH)3 |
| Melting Point | 135 °C |
| Density | 4.25 g/cm³ |
| Molar Mass | 106.867 g/mol |
| Solubility in Water | Insoluble |
Iron III Hydroxide Chemical Structure
As a compound composed of iron (Fe) ions bonded to hydroxide (OH) ions.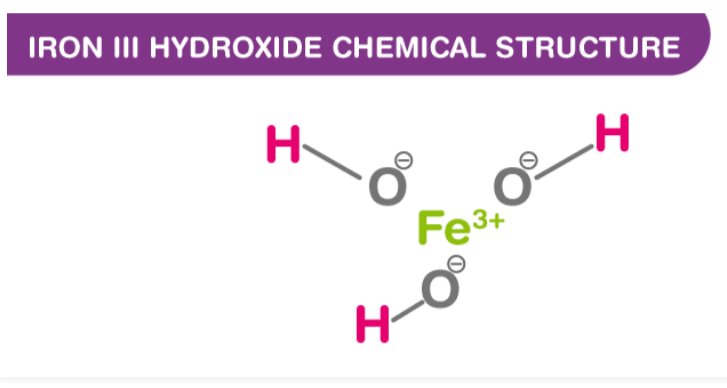 In this representation, the Fe atom is at the center, bonded to three OH groups (hydroxide ions), bonded to hydrogen (H) atoms. Hydroxide ions consist of one hydrogen atom and one oxygen atom bonded together. An iron(III) hydroxide solid usually has a brownish-red appearance and is commonly found in nature as a variety of minerals.
In this representation, the Fe atom is at the center, bonded to three OH groups (hydroxide ions), bonded to hydrogen (H) atoms. Hydroxide ions consist of one hydrogen atom and one oxygen atom bonded together. An iron(III) hydroxide solid usually has a brownish-red appearance and is commonly found in nature as a variety of minerals.
Also Read: Aluminium Acetate Formula
Properties of Iron III Hydroxide
Below are some of the main properties of Iron III Hydroxide:- The color of Iron(III) Hydroxide can vary from yellow to dark brown to black, based on its degree of hydration, particle size and shape, and crystal structure.
- The formula for Iron Hydroxide is Fe(OH)3.
- The molar mass of this compound is 106.867g/mol.
- Iron III Hydroxide has a melting point of 135 degrees Celsius and a 4.25 grams per cubic centimeter density.
- Water is insoluble Iron III Hydroxide.
Also Check - Molar Volume formula
Preparation of Iron III Hydroxide
Sodium Hydroxide (NaOH) was added to an iron (III) ion-containing solution in order to test for the presence of positive metal ions in the solution and form a rust-brown gelatinous solid known as Iron (III) Hydroxide precipitate. This technique allowed us to simulate similar conditions found at the redox boundary of natural waters by slowly generating the precipitant Iron (III) through oxygenation of Iron (II) and hydrolysis.Also Check - Periodic Acid formula
Natural Occurrences
Bernalite, an exceedingly rare mineral with the chemical formula Fe(OH)3·nH2O (with n values ranging from 0.0 to 0.25), is an example of an anhydrous ferric hydroxide. FeOOH, or iron oxyhydroxides, are more prevalent and can form into various structural minerals, known as polymorphs, using the Greek labels α, β, γ, and δ. For instance, goethite (α-FeO(OH)), has been used as a yellow-brown pigment since prehistoric times. Lastly, feroxyhyte (δ) will form under high-pressure conditions found on ocean floors and is not thermodynamically stable compared to the alpha polymorph (or goethite).Also Check - Aluminium Acetate Formula
Uses of Iron III Hydroxide Formula
- Limonite, a combination of polymorphs and hydrates of ferric oxyhydroxide, is one of the oldest iron ores known to have been utilized since 2500 BC.
- Pigment Yellow 42, another name for Yellow iron oxide, has been approved by the Food and Drug Administration for cosmetics and tattoo inks.
- Additionally, nanoparticles of iron oxide-hydroxide have been used in aquarium water treatment as a phosphate binder and investigated as an adsorbent for removing lead from aquatic media.
Iron III Hydroxide Safety Hazards
- The possible risks associated with Iron III Hydroxide include inhalation of airborne particles which can irritate the eyes and/or respiratory tract. Short-term exposure could lead to further effects.
- When storing Iron Hydroxide, it is recommended to keep it away from heat sources and combustible materials, as temperatures above 356°F (180°C) may cause a gradual colour change in the product.
- Furthermore, contact with strong oxidising agents should be avoided.
<span style=
What is Iron III Hydroxide?
Is Iron III Hydroxide soluble in water?
What are the common uses of Iron III Hydroxide?
Is Iron III Hydroxide hazardous to health?
How can Iron III Hydroxide be synthesized?










