
Percentage Yield Formula: The percentage yield is a measure of the efficiency of a chemical reaction. It tells you how much of the desired product was actually obtained in a reaction compared to the theoretical maximum amount that could be obtained. The formula for calculating percentage yield is:
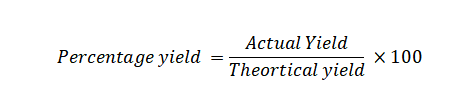
Where:
Actual Yield is the amount of product you actually obtained from the reaction.
Theoretical Yield is the maximum amount of product that could theoretically be obtained based on stoichiometry and the amount of reactants used.
Also Check - Percent by Weight Formula
Percentage Yield Formula Solved Examples
Here's a solved example to illustrate how to calculate percentage yield:
Example 1: What is the percentage yield when conducting a chemical reaction with an expected yield of 50 grams of a certain compound based on stoichiometry, but the actual yield obtained during the reaction is only 42 grams?
Actual Yield: 42 grams
Theoretical Yield: 50 grams (as expected)
Next, insert these values into the formula:
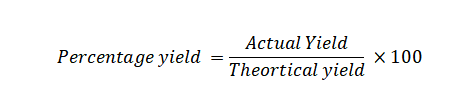
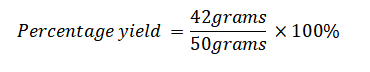
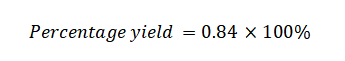
Percentage Yield=84%
So, the percentage yield of the reaction is 84%. This means that you obtained 84% of the maximum possible amount of the product based on the stoichiometry of the reaction.
Also Check - Partial Pressure Formula
Example 2: What is the percentage yield when attempting to synthesize a compound with a theoretical yield of 120 grams, but obtaining only 95 grams of the compound during the actual experiment?
Actual Yield: 95 grams
Theoretical Yield: 120 grams
Using the percentage yield formula:
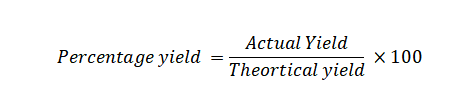
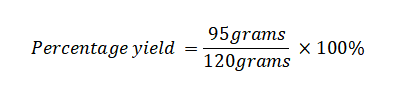
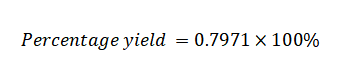
Percentage Yield=79.17%
The percentage yield in this case is approximately 79.17%.
Also Check - Net Ionic Formula
Example 3: What is the percentage yield when conducting a chemical reaction with an expected yield of 60 moles of a compound but obtaining only 48 moles of the compound?
Actual Yield: 48 moles
Theoretical Yield: 60 moles
Using the percentage yield formula:
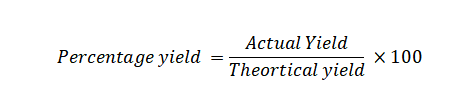
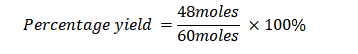
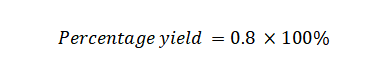
Percentage Yield= 80%
In this case, the percentage yield is 80%, indicating that you obtained 80% of the maximum possible amount of the compound based on stoichiometry.
Example 4: What is the percentage yield if you conduct an experiment aiming to produce 25 grams of a chemical compound but only obtain 22 grams of the compound after the experiment?
Actual Yield: 22 grams
Theoretical Yield: 25 grams
Using the percentage yield formula:
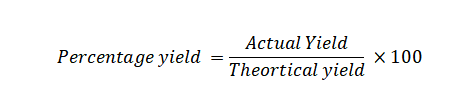
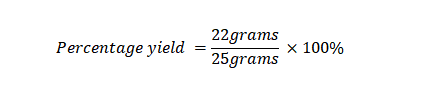
Percentage Yield=88%
So, the percentage yield in this case is 88%.
Example 5: What is the percentage yield when you are in the process of synthesizing a chemical compound, and your initial expectation is to produce 180 moles of the compound, but the actual yield obtained from the synthesis is only 162 moles?
Actual Yield: 162 moles
Theoretical Yield: 180 moles
Using the percentage yield formula:
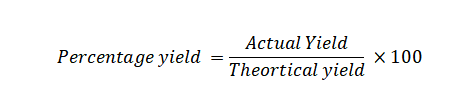
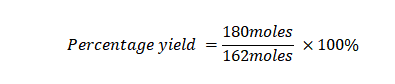
Percentage Yield=90%
In this case, the percentage yield is 90%, indicating that you obtained 90% of the maximum possible amount of the compound based on stoichiometry.
These examples illustrate how to calculate percentage yield in various situations, whether you are dealing with grams or moles of a substance.
The percentage yield formula is applied to measure how efficiently a chemical reaction produces a desired product. It compares the actual amount of product obtained in an experiment to the maximum amount that should theoretically be produced based on the balanced chemical equation. It helps chemists evaluate the success of their reactions and make improvements if the yield is lower than expected.
P<span style=
What is the Percentage Yield Formula?
How do you calculate Percentage Yield?
Why is Percentage Yield important in chemistry?
What does a Percentage Yield of 100% mean?










