
The relative lowering of vapour pressure is crucial in chemistry, especially when dealing with dilute solutions. This phenomenon falls under the category of colligative properties, where the properties of a solution depend on the number of solute particles rather than the nature of the solute itself. Let's delve into the formula, its implications, and the specific case of a dilute glucose solution.
Relative Lowering of Vapour Pressure
Vapour pressure is the pressure exerted by vapours at equilibrium over a liquid, which depends on temperature. For instance, if we consider a pure liquid, its molecules occupy the surface. However, if we introduce a non-volatile solute into this pure liquid, the resulting vapour above the solution will only contain molecules of the solvent (pure liquid). As a result, there will be a decrease in the vapour pressure of the solution compared to that of the pure liquid at the same temperature. The decrease in vapour pressure can be attributed to the addition of the solute to the pure liquid (solvent), resulting in a surface that consists of both solvent and solute molecules. As a result, there is a decrease in the number of solvent molecules escaping into the vapour phase, leading to a reduction in vapour pressure. This phenomenon, known as relative lowering of vapour pressure, is a colligative property that is dependent on the amount of non-volatile solute added to the solution, regardless of its composition.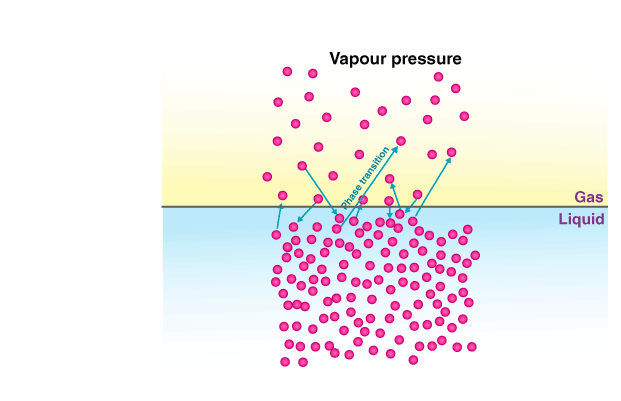
The formula for Relative Lowering of Vapour Pressure
The relative lowering of vapor pressure ) can be calculated using the formula: = P pure solvent − P solution Where: P pure solvent is the vapor pressure of the pure solvent. P solution is the vapor pressure of the solution.Dependence of Relative Lowering of Vapor Pressure
It is important to note that the relative lowering of vapor pressure depends solely on the number of solute particles in the solution, not on the nature of the solute itself. This is a fundamental principle of colligative properties.Colligative Property Significance
Colligative properties, such as relative lowering of vapor pressure, are affected by the concentration of solute particles in a solution. In this case, the more solute particles present, the greater the decrease in vapor pressure. This property is independent of the identity of the solute molecules; it could be any non-volatile solute.Relative Lowering of Vapor Pressure in Dilute Glucose Solution
Considering a dilute solution of glucose, the relative lowering of vapor pressure ( Δ P glucose ) can be expressed as: Δ P glucose =K f ×molality Here, K f is the cryoscopic constant, a property of the solvent, and molality represents the concentration of the solute.Dimensionless Quantity
The relative lowering of vapor pressure is a dimensionless quantity, as it is a ratio of vapor pressures ( Δ P solvent / P pure solvent ). This makes it a convenient measure for comparing different solutions. In conclusion, understanding the relative lowering of vapor pressure and its formula is crucial in predicting and explaining the behavior of solutions. This concept, along with other colligative properties, provides valuable insights into the physical properties of solutions, offering a bridge between macroscopic observations and microscopic interactions at the molecular level.| Related Links | |
| Cyanide Formula | Copper Formula |
| Carbonous Acid Formula | Cholesterol Formula |
Relative Lowering of Vapour Pressure FAQs
What is the relative lowering of vapour pressure?
Relative lowering of vapour pressure is a colligative property of solutions. It quantifies the decrease in vapour pressure of a solvent when a non-volatile solute is added and is expressed as the difference between the vapour pressure of the pure solvent and the vapor pressure of the solution.
What is the relationship between the relative lowering of vapour pressure and osmotic pressure?
The relationship lies in their both being colligative properties. Relative lowering of vapor pressure and osmotic pressure are interconnected through formulas involving constants such as the gas constant and temperature, reflecting the impact of solute concentration on these properties in a solution.
What is the connection between the relative lowering of vapour pressure and Raoult's Law?
Raoult's Law is closely related to the relative lowering of vapor pressure as it describes ideal behaviour in solutions. According to Raoult's Law, the vapor pressure of a solution is proportional to the mole fraction of each component. The relative lowering of vapor pressure is a measure of the deviation from ideal behavior in non-ideal solutions.
Why is relative lowering in vapour pressure called a colligative property?
Relative lowering of vapour pressure is termed a colligative property because it depends solely on the number of solute particles in a solution, not on the nature of the solute. This property is a consequence of the collective behavior of solute particles impacting the physical properties of the solvent, making it a key concept in understanding the properties of dilute solutions.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2025 Physicswallah Limited All rights reserved.









