
Derived from Greek words meaning bile, solid, and alcohol, Cholesterol is an organic compound known as a sterol. It serves as a structural component of animal cell membranes and can be produced by all animals through biosynthesis. Other names for cholesterol include cholesteryl alcohol and cholesterin. Its properties include a hydrogen bond donor value of 1, acceptor value of 1, and 5 rotatable bonds. In addition to its role in the cell membrane, cholesterol is a precursor for steroid hormones, bile acid, and vitamin D biosynthesis. It is the most common sterol found in animals and is primarily generated by hepatic cells in vertebrates. While it is necessary for growth in Mycoplasma bacteria, prokaryotes such as bacteria and archaea do not contain cholesterol. This article will examine its formula, chemical name, structure and function.
Properties Of Cholesterol
| IUPAC name | (3β)-cholest-5-en-3-ol |
| Molecular formula | C 27 H 46 O |
| Molecular mass | 386.664 g/mol |
| Melting point | 148 to 150℃ |
| Boiling point | 360℃ |
| Density | 1.052 g/cm3 |
| Solubility | Soluble in acetone, benzene, ether, hexane |
| Solubility in water | 1.8 mg/L at 30℃ |
| Appearance | White crystalline powder |
Structure of Cholesterol
It plays the role of a human metabolite, mouse metabolite, algal metabolite and Daphnia galeata metabolite as cholesterol is a cholestanoid with a double bond at 5,6-position and a 3beta-hydroxy group. It is a 3beta-sterol, a cholestanoid, a 3beta-hydroxy-delta(5)-steroid and a C27-steroid.
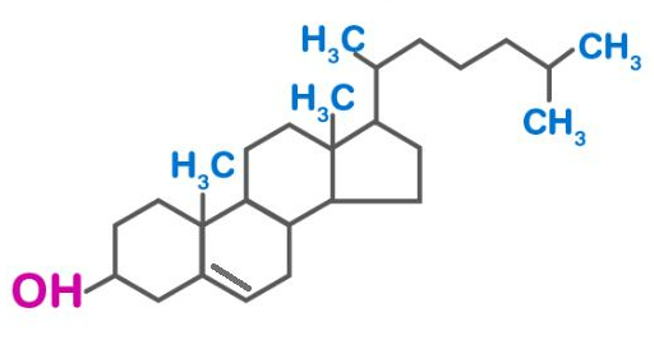
As a waxy substance, cholesterol can be found in both plasma and animal tissues. Chemically, cholesterol belongs to the steroid family. It is a white, crystalline substance that is odourless and tasteless. It forms the main component of the membrane surrounding each cell. It is the starting material for the body to synthesize bile acids, steroid hormones, and vitamin D.
As a result of a traditional diet, the citizens also consume substantial amounts of cholesterol. Cholesterol circulates throughout the bloodstream and several other organs. As part of compensatory system regulation, the liver synthesizes a higher amount of cholesterol. A higher dietary intake of cholesterol reduces liver synthesis of the compound, and it's also a precursor to all steroid hormones and vitamin D.
Also Check – Ammonium Nitrate Formula
Biosynthesis
Acetoacetyl-CoA is made from the condensation of two molecules of acetyl CoA within the body through the mevalonate pathway. A second condensation occurs between acetyl CoA and acetoacetyl-CoA to form 3-hydroxy-3-methylglutaryl CoA (HMG-CoA). This molecule is then reduced to mevalonate by the enzyme HMG-CoA reductase.
A phosphorylation step and a decarboxylation step involving ATP are required to convert mevalonate to isopentenyl pyrophosphate (IPP). Geranyl transferase condenses three molecules of isopentenyl pyrophosphate into farnesyl pyrophosphate. The endoplasmic reticulum then converts two molecules of farnesyl pyrophosphate into squalene by the action of squalene synthase.
Also Check – Chemistry Formula For Solubility Product
After oxidizing squalene, oxysqualene cyclase produces lanosterol. Lanosterol is converted into cholesterol by NADPH and oxygen, which help oxidize methyl groups in order to remove carbons, mutases in order to move alkene groups, and NADH in order to reduce ketones. Through homeostatic mechanisms, cholesterol levels directly affect cholesterol biosynthesis.
Cholesterol Formula FAQs
Q1. What is the chemical formula of cholesterol?
Q2. What is the primary function of cholesterol in the body?
Q3. Is all cholesterol harmful to health?
Q4. What factors influence cholesterol levels in the body?
Q5. Can you have too little cholesterol in your body?










