
Sodium Dichromate Formula: Sodium dichromate, represented by the formula Na 2 Cr 2 O 7 , It is usually handled as its dihydrate, Na 2 Cr 2 O 7 ·2H 2 O. Most chromium ore is transformed into sodium dichromate, serving as a precursor for various chromium-based compounds and materials. In terms of reactivity and appearance, sodium dichromate closely resembles potassium dichromate, but it boasts significantly higher solubility in water and a lower equivalent weight, which can be advantageous in various applications.
Sodium dichromate serves as the primary source material for a variety of chromium compounds used in diverse applications, such as leather tanning, metal treatment, drilling muds, textile dyes, catalysts, and wood and water treatment.
What is Sodium Dichromate?
Sodium dichromate formula is Na 2 Cr 2 O 7. It is an orange-red inorganic crystalline compound known for emitting toxic chromium fumes upon heating. It exhibits strong corrosive properties and serves as a potent oxidizer. This chemical finds common use in the synthesis of various chromium compounds and is referred to by alternative names like bichromate of soda, disodium dichromate, or sodium dichromate (VI).
Sodium Dichromate Formula Name
Sodium dichromate is a solid crystalline material with a red to orange color. It belongs to the category of chemicals referred to as chromium (VI) compounds. This compound is also known by several alternate names, such as sodium bichromate, bichromate of soda, and sodium dichromate (VI).
Sodium Dichromate Formula Charge
The sodium dichromate formula is Na 2 Cr 2 O 7 . Within this compound, each sodium (Na) atom carries a positive charge of +1, while the dichromate (Cr 2 O 7 ) ion bears a negative charge of -2.
Sodium Dichromate Structure
The chemical compound Na 2 Cr 2 O 7 is known as Sodium dichromate. It is also known as Bichromate of soda, Disodium dichromate, or Sodium dichromate (VI). This substance is a potent oxidizing agent and extremely corrosive. Sodium dichromate (VI) appears as a red to red-orange crystalline solid.
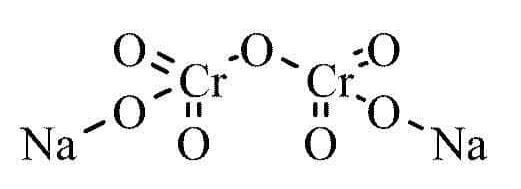
Preparation of Sodium Dichromate
On a larger scale, sodium dichromate is obtained from metal ores containing chromium (III) oxides. This process is conducted at a temperature of 1000°C in the presence of oxygen, resulting in the following reaction:
2Cr 2 O 2 + 4Na 2 CO + 3O 2 → 4Na 2 CrO 4 + 4CO 2
This reaction proceeds further as follows:
2Na 2 CrO 4 + 4CO 2 + H 2 O → Na 2 CrO 7 + 2NaHCO 3
2Na 2 CrO 4 + H 2 SO 4 → Na 2 Cr 2 O 7 + Na 2 SO 4 + H 2 O
Through a crystallization process, dichromate is isolated as a dihydrate.
Sodium Dichromate Formula Physical Properties
- Sodium Dichromate has a molecular weight of 262 g/mol.
- Its density is 2.52 g/cm3.
- The boiling point of Sodium Dichromate is 400°C.
- Sodium Dichromate is odorless.
- It has a refractive index of 1.66.
- The chemical formula of Sodium Dichromate is Na 2 Cr 2 O 7 .
Sodium Dichromate Formula Chemical Properties
The chemical formula for sodium dichromate is Na 2 Cr 2 O 7 . Here are some of its chemical properties:
Oxidizing Agent: Sodium dichromate is a powerful oxidizing agent, meaning it has the ability to cause other substances to undergo oxidation reactions.
Solubility: It is soluble in water and can dissolve in various solvents.
Acidic Nature: In aqueous solutions, sodium dichromate is acidic and can release chromic acid (H 2 CrO 4 ).
Reactivity: It is reactive with various other chemicals and can participate in redox reactions, especially with reducing agents.
Chromium Compounds: Sodium dichromate is a precursor for various chromium compounds and materials.
Corrosive: It is highly corrosive and should be handled with care.
Toxicity: It is toxic and poses health risks if not handled properly.
Color: Sodium dichromate typically appears as an orange-red to red crystalline solid.
Environmental Concerns: It is considered an environmental hazard due to the toxicity of chromium compounds, and its disposal and use are regulated in many places to prevent environmental contamination.
Uses of Sodium Dichromate
Sodium dichromate plays a crucial role in the production of chromium compounds.
It finds use in petroleum refining.
It is used for water treatment purposes.
Sodium dichromate serves as an oxidizing agent in the production of chemical compounds.
Its primary function lies as a catalyst.
Sodium dichromate is used as an insecticide and fungicide.
It is applied in colorimetry experiments to determine copper content.
Sodium dichromate is instrumental in creating colored glass and ceramic frosting.
It is essential for producing inorganic chromate pigments that yield light-stable hues.
It is utilized in leather tanning processes.
| Related Links | |
| Fluorine Gas Formula | Potassium Hexacyanoferrate (III) Formula |
| Potassium Fluoride Formula | Potassium Chlorate Formula |
Sodium Dichromate Formula FAQs
What is the chemical formula for Sodium Dichromate?
What are some alternative names for Sodium Dichromate?
What is the charge of Sodium and the Dichromate ion in Sodium Dichromate?
What is the color of Sodium Dichromate?
What are the physical properties of Sodium Dichromate?










