
Potassium Hexacyanoferrate III Formula: Potassium hexacyanoferrate (III), also known as Red prussiate of Potash or Potassium ferricyanide with the chemical formula K 3 [Fe(CN) 6 ], was first identified by Leopold Gmelin in 1822. This compound consists of potassium, iron, carbon, and nitrogen elements, manifesting as deep red crystals that decompose when exposed to heat. Within its structure, it contains the octahedrally coordinated [Fe(CN) 6 ] 3− ion.
It is soluble in water and its solution exhibits a subtle green-yellow fluorescence. When subjected to a strong acid, it releases highly toxic hydrogen cyanide gas. The synthesis of potassium ferricyanide involves passing chlorine through a solution of potassium ferrocyanide.
This compound finds extensive application in photography and blueprint drawing, as well as in calico printing, electroplating, and wool dyeing. It is also employed as a laboratory reagent and functions as an oxidant in organic chemistry. Notably, it plays a crucial role in the preparation of Murakami's etchant, which is utilized by metallographers.
Potassium Hexacyanoferrate III Formula Structure
The Potassium Hexacyanoferrate III formula is K 3 [Fe(CN) 6 ]. Its structure includes an octahedrally coordinated [Fe(CN) 6 ] 3− ion.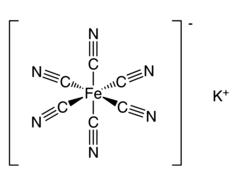
Preparation of Potassium Hexacyanoferrate III
To produce Potassium Hexacyanoferrate III, one can pass chlorine through a solution of potassium ferrocyanide, leading to the following chemical reaction:
2K 4 [Fe(CN) 6 ] + C l2 → 2K 3 [Fe(CN) 6 ] + 2KClPotassium Hexacyanoferrate III Formula Physical Properties
Here are some key physical characteristics of Potassium Hexacyanoferrate (III):
Chemical Formula: Potassium Hexacyanoferrate III Formula is K 3 [Fe(CN) 6 ]
Other Names: Potassium ferricyanide, Red prussiate of Potash, Prussian red
Molar Mass: 329.24 g/mol
Appearance: Deep red crystals
Density: 1.89 g/cm3
Melting Point: 300 °C
Boiling Point: Decomposes
Solubility: Soluble in water, acid, and slightly soluble in alcohol
Potassium Hexacyanoferrate III Formula Chemical Properties
Potassium Hexacyanoferrate (III) exhibits various chemical reactions, as outlined below: Reaction with a Strong Acid: When Potassium Hexacyanoferrate (III) reacts with a strong acid, it yields highly toxic hydrogen cyanide gas: 6H+ + [Fe(CN)6]3− → 6HCN + Fe3+ Formation of Potassium Ferrocyanide: When Potassium Hexacyanoferrate (III) reacts with potassium hydroxide, it leads to the formation of potassium ferrocyanide: 4KOH + 4K 3 [Fe(CN) 6 ] → 2H 2 O + O 2 + 4K 4 [Fe(CN) 6 ] Reaction with Water: In a boiling solution, Potassium Hexacyanoferrate (III) reacts with water, producing potassium monopentacyanoferrate and potassium cyanide: K 3 [Fe(CN )6 ] + H 2 O → K 2 [Fe( H2 O)(CN )5 ] + KCN Reaction with Iron (III) Chloride: When Potassium Hexacyanoferrate (III) interacts with iron (III) chloride, it generates iron (III) hexacyanoferrate (III) and potassium chloride: K 3 [Fe(CN) 6 ] + FeCl 3 → Fe[Fe(CN) 6 ] + 3KCl Formation of Turnbull's Blue: The reaction of Potassium Hexacyanoferrate (III) with freshly prepared iron (II) sulfate solution results in the formation of a dark blue precipitate called Turnbull's blue: 4K 3 [Fe(CN) 6 ] + 3FeSO 4 → Fe 4 [Fe(CN) 6 ] 3 + 3K 2 SO 4 + 6KCN Reaction with Lead (II) Hydroxide and Potassium Hydroxide Solution: When Potassium Hexacyanoferrate (III) interacts with lead (II) hydroxide and a diluted potassium hydroxide solution, it yields potassium hexacyanoferrate (II), lead (IV) oxide, and water: 2K 3 [Fe(CN) 6 ] + 2KOH + Pb(OH) 2 → 2K 4 [Fe(CN) 6 ] + PbO 2 + 2H 2 OHealth Hazards of Potassium Hexacyanoferrate III
Harmful if Swallowed or Inhaled: Potassium Hexacyanoferrate III can be harmful if ingested or inhaled.
Low Toxicity with Mild Irritation: While it has relatively low toxicity, it may cause mild irritation to the eyes, skin, and respiratory tract.
Irritating or Toxic Fumes on Heating: When exposed to heat, this compound can release fumes that are either irritating or toxic
Uses Potassium Hexacyanoferrate III
Mild Oxidizing Agent in Organic Chemistry: This compound serves as a gentle oxidizing agent in organic chemistry.
Calico Printing and Wool Dyeing: It finds utility in the fields of calico printing and wool dyeing.
Electroplating: In the process of electroplating, this compound plays a significant role.
Photography and Blueprint Drawing: It is widely utilized in photography and blueprint drawing.
Iron and Steel Hardening: Potassium Hexacyanoferrate III is used to harden iron and steel.
Laboratory Reagent: It is employed as a reagent in laboratory experiments.
| Related Links | |
| Potassium Fluoride Formula | Potassium Chlorate Formula |
| Potassium Bromate Formula | Gold Formula |
Potassium Hexacyanoferrate III Formula FAQs
What is the chemical formula of Potassium Hexacyanoferrate III?
What are the common names for Potassium Hexacyanoferrate III?
What is the molar mass of Potassium Hexacyanoferrate III?
What are some common uses of Potassium Hexacyanoferrate III?
What is the IUPAC nomenclature for K3[Fe(CN)6]?










