
Fluorine Gas Formula: Fluorine, denoted by the symbol F with an atomic number of 9 on the periodic table, is the lightest halogen and exists in a diatomic, highly toxic gaseous state under standard conditions. It exhibits a pale yellow color and possesses exceptional electronegativity. Let's discuss the formula, occurrence, and properties of fluorine gas.
Fluorine Gas Formula
Fluorine gas is represented by the chemical formula F 2 , also known as Difluoride due to its composition of two fluorine (F) atoms. It reacts with nearly all elements, with the exceptions of argon, neon, and helium. In its gaseous form, it appears pale yellow, while in the liquid state, it displays a bright yellow hue. In the solid state, fluorine is transparent and opalescent, existing in two distinct forms referred to as alpha and beta.Fluorine Gas Formula Lewis Structure
The chemical formula for fluorine gas is F 2 , meaning it consists of two fluorine (F) atoms bonded together. When representing the Lewis structure for fluorine gas (F2), you would depict it as follows:
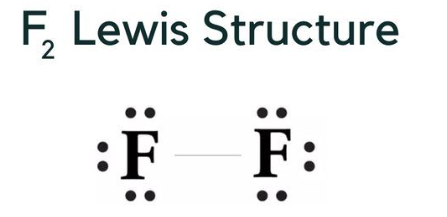
In this Lewis structure, each "F" represents a fluorine atom, and the line between them represents a covalent bond, indicating the sharing of electrons between the two fluorine atoms. Each fluorine atom has seven valence electrons, and in this diatomic molecule, they share one electron with each other to form a single covalent bond.
Diatomic Fluorine Gas Formula
Diatomic fluorine gas has the chemical formula F 2 . This means it consists of two fluorine (F) atoms chemically bonded together to form a molecule of F 2 .Fluorine Gas Formula and Charge
Fluorine gas (F 2 ) is a diatomic molecule composed of two fluorine (F) atoms. Each fluorine atom in the molecule has a charge of -1 because it gains one electron to achieve a full outer electron shell (valence shell) of eight electrons, similar to the noble gas configuration. When two fluorine atoms come together to form the F 2 molecule, they share electrons through a covalent bond, resulting in a stable compound with no net charge. So, fluorine gas (F 2 ) itself does not have an overall charge, it is a neutral molecule.Preparation of Fluorine Gas
Fluorine is primarily found in the form of fluoride compounds. The production of hydrogen fluoride (HF) is achieved by combining calcium fluoride (CaF 2 ) and sulfuric acid (H 2 SO 4 ) through the following chemical reaction:
CaF 2 + H 2 SO 4 → 2HF + CaSO 4 .
Alternatively, fluorine gas can be generated by the hydrolysis of hexafluorosilicic acid (H2SiF6), resulting in the release of hydrogen fluoride (HF):
H 2 SiF 6 → 2HF + SiF 4 .
SiF 4 + 2H 2 O → 4HF + SiO 2 .
Fluorine Gas Formula Physical Properties
The molar mass of fluorine gas is approximately 18.998403 g/mol.
It has a very low melting point at -219.67°C.
The boiling point of fluorine gas is -188.11°C.
It exhibits a density of 1.8 x 10^-3 g/cm³.
Fluorine gas readily forms compounds with metals, non-metals, metalloids, and even noble gases.
This element is reactive with both glass and water.
Fluorine Gas Formula Chemical Properties
When fluorine interacts with water (H 2 O), it produces hydrogen fluoride (HF) and releases oxygen gas (O 2 ).
2F 2 + 2H 2 O → 2HF + O 2 .
In the presence of sodium hydroxide (NaOH), a reaction with fluorine results in the formation of sodium fluoride (NaF), oxygen difluoride (OF 2 ), and water (H 2 O).
F 2 + 2NaOH → 2NaF + OF 2 + H 2 O.
Uses of Fluorine Gas
It is used in the preparation of uranium hexafluoride (UF6) for applications in the nuclear fuel cycle.
Fluorine gas is used for fluorinating uranium tetrafluoride.
Fluorine gas serves the purpose of a potent oxidizing agent in various chemical processes.
It is utilized in the production of light bulbs.
Fluorine gas plays a crucial role in the manufacture of Teflon.
The compound finds utility in the toothpaste industry due to its effectiveness in preventing dental cavities.
| Related Links | |
| Potassium Hexacyanoferrate (III) Formula | Potassium Fluoride Formula |
| Potassium Chlorate Formula | Potassium Bromate Formula |
Fluorine Gas Formula FAQs
What is the formula of fluorine gas?
How is fluorine gas represented in its Lewis structure?
Is fluorine gas a diatomic molecule?
What is the charge of each fluorine atom in fluorine gas (F2)?










