Potassium Chlorate Formula: Potassium is represented by the chemical symbol K, with an atomic number of 19. Its electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 . This element, found in the first group of the periodic table and belonging to the fourth period, exhibits a silvery-white appearance. It occurs naturally in mineral deposits and salts. Potassium plays a crucial role in nerve function and muscle contraction. It is most commonly employed for the treatment and prevention of low potassium levels. The credit for its discovery in 1807 goes to Sir Humphry Davy.
Chlorine, symbolized as Cl and possessing an atomic number of 17, has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 5 . This element is widely utilized as an antiseptic to combat bacteria and ensure safe drinking water. Chlorine finds application in various industrial processes, including the production of paper, plastics, textiles, and medicines.
Oxygen, denoted by the symbol O, has an atomic number of 8 and an electron configuration of 1s 2 2s 2 2p 4 . As a member of the chalcogen group in the periodic table, it exists as an odorless, colorless, and tasteless diatomic gas, comprising 21% of Earth's atmosphere. Oxygen is indispensable for the process of respiration and has essential applications in refining, melting, and manufacturing steel, as well as in the production of various other metals.
Potassium Chlorate Formula
Potassium Chlorate, an inorganic compound with the molecular formula KCLO 3 , consists of one potassium atom, one chlorine atom, and three oxygen atoms. It serves as a robust oxidizing agent and presents as a white crystalline solid. In industrial applications, it ranks as the second most commonly used chlorate. This crystalline salt exhibits a cooling saline taste, particularly employed in veterinary medicine as a mild astringent. It surpasses potassium perchlorate in oxidizing capabilities and is a prevalent choice for oxidizers in pyrotechnics. Typically, potassium chlorate is synthesized from household bleach and salt substitutes.Potassium Chlorate Formula Structure
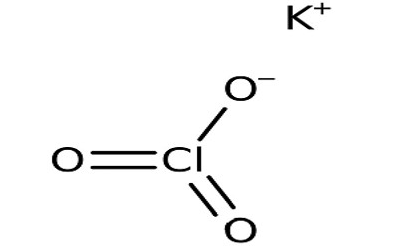
The Potassium Chlorate formula is KClO 3 . Its molecular structure consists of one potassium (K) atom, one chlorine (Cl) atom, and three oxygen (O) atoms. The atoms are arranged in the molecule as follows:
 [caption id="attachment_45231" align="alignnone" width="300"]
[caption id="attachment_45231" align="alignnone" width="300"]
 Potassium Chlorate Formula Structure[/caption]
Potassium Chlorate Formula Structure[/caption]
In this structural representation, the potassium atom (K) is bonded to the chlorine atom (Cl) and three oxygen atoms (O) through chemical bonds. This arrangement forms the potassium chlorate molecule.
Potassium Chlorate Formula Charge
Compound KClO 3 has a neutral charge (0), and potassium (K) carries a charge of +1, the ClO 3 group must have a combined charge of -1 to balance out the potassium's +1 charge, resulting in an overall neutral charge (0) for the compound.Potassium Chlorate Formula Physical Properties
Molar mass: 122.55 gm/mol
Density: 2.34 gm/cm3
Melting point: 400°C
Boiling point: 356°C
Potassium Chlorate Formula Chemical Properties
Potassium chlorate is a colorless solid and readily soluble in water.
It dissolves in glycerol but does not dissolve in many organic solvents.
It exhibits compatibility with various polar solvents.
When subjected to heat in the presence of a manganese dioxide catalyst, it undergoes decomposition, yielding potassium chloride and oxygen gas as follows:
2 KCLO 3 → 2 KCL + 3 O 2 In the presence of sugar and sulfuric acid, potassium chlorate undergoes a vigorous reaction, releasing a significant amount of heat energy, resulting in a purplish flame and substantial smoke production: C 12 H 22 O 11 + 8 KCLO 3 → 12 CO 2 + 11 H 2 O + 8 KCL + heatUses of Potassium Chlorate
Potassium Chlorate finds extensive use as an oxidizing agent in various industries, including explosives, matches, textile printing, and bleaches.
It plays a role in pharmaceuticals and veterinary medicine.
Both as an antiseptic and an astringent, potassium chlorate serves in medical applications.
It is a popular choice as an oxidizer in pyrotechnics, contributing to vibrant fireworks displays.
Additionally, potassium chlorate is utilized in the production of oxygen gas for various industrial purposes.
| Related Links | |
| Gold Formula | Phosphate Formula |
| Helium Formula | Hydrazine Formula |
Potassium Chlorate Formula FAQs
What is the chemical formula of Potassium Chlorate?
What is the molecular structure of Potassium Chlorate?
What are the physical properties of Potassium Chlorate?
Why is potassium chlorate banned?