

Sodium Formate Formula , a simple but remarkable chemical compound, finds applications in various fields due to its unique properties. Sodium, with atomic numbers ranging from 2 to 38 and the symbol Na, is a highly reactive alkali metal found abundantly in the Earth's crust. It ranks as the 11th most common element on Earth, after water, coal, iron, oxygen, nitrogen, and carbon. It can be found in minerals such as feldspar and rock salt (NaCl), and many of its salts are known for their strong hygroscopic properties. Sodium chloride (NaCl) has a similar appearance to water when found in mineral form and is one of the main components of seawater by weight.
Sodium Formate Structure
Sodium formate has a straightforward and symmetrical structure. It consists of a sodium cation (Na+) and a formate anion (HCOO-). The formate anion is composed of one carbon atom (C) bonded to two oxygen atoms (O), one hydrogen atom (H), and an extra hydrogen atom (H) that serves as the ionic bond to sodium (Na+).
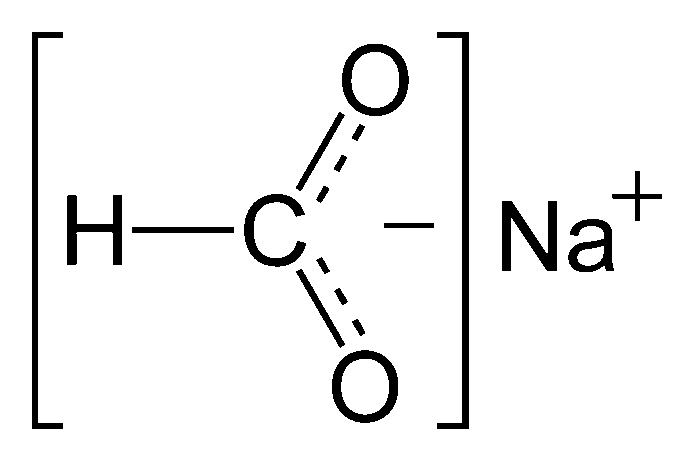
Sodium Formate Buffer Formula
Sodium formate is often used as a component in buffer solutions. A standard buffer formula using sodium formate involves mixing it with formic acid (HCOOH), resulting in a buffer system with specific pH-regulating capabilities. The specific formula may vary depending on the desired pH.
Sodium Formate Acid Formula
Sodium formate can behave as an acid when dissolved in water. Its acid formula corresponds to its ionization in an aqueous solution. It can donate a hydrogen ion (H+), resulting in the formate anion (HCOO-) acting as a weak acid. The dissociation equation is: NaHCOO ⇌ Na+ + HCOO-
Also Check – Percentage Yield Formula
Sodium Formate Compound Formula
The compound formula for sodium formate is NaHCOO, reflecting its composition as an ionic compound composed of sodium (Na+) and formate (HCOO-) ions.
Sodium Formate Salt Formula
Sodium formate can be considered a salt, as it is an ionic compound formed from the reaction of a metal (sodium) and a non-metal (formate). Its salt formula is NaHCOO, representing its ionic nature.
Also Check – Percent by Weight Formula
Sodium Formate in Water Equation
When sodium formate dissolves in water, it undergoes ionisation, releasing sodium cations (Na+) and formate anions (HCOO-). The equation for this process is: NaHCOO → Na+ + HCOO-
Sodium Formate: Acid or Base?
Sodium formate can exhibit amphoteric behaviour, acting as an acid and a base. Its acidic properties are evident when it donates a hydrogen ion (H+) to a solution, while its essential characteristics become apparent when it accepts a hydrogen ion. This versatility makes it valuable in various applications, such as pH regulation and a buffering agent.
Also Check – Gibbs Free Energy Formula
Uses of Sodium Formate
- Sodium Formate Formula can be used as a buffering agent.
- It is also used for deicing runways of airports.
- Sodium Formate is used in Printing processes.
- There are also applications for Sodium Formate Formula in pigmenting and cloth dyeing industries.
Sodium Formate Formula FAQs
Q1. What is the formula of sodium formate?
Q2. What is the formula for sodium formate in water?
Q3. What is sodium formate also known as?
Q4. Why is sodium formate basic?












