Sulfur Trioxide Formula: Sulfur trioxide, denoted as SO3, is a chemical compound characterized by the molecular formula SO3. It exists in various forms, each possessing unique molecular structures and crystalline arrangements. In its liquid state, SO3 is transparent and releases vapors under typical conditions. This chemical compound is remarkably reactive, functioning both as a potent oxidizing agent and a fire hazard. When compared to selenium dioxide, it displays a thermodynamic instability.
Also Check – S urface Chemistry Formula
Structure of Sulfur Trioxide
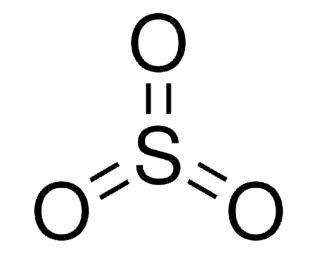
SO3, in its gaseous state, takes on a trigonal planar shape with D3h symmetry, as anticipated by the VSEPR (Valence Shell Electron Pair Repulsion) theory. As a consequence, SO3 is categorized within the D3h point group.
The sulfur atom holds an oxidation state of +6 and carries no formal charge according to electron-counting principles. In its Lewis structure, SO3 exhibits one S=O double bond and two S–O dative bonds.
The gaseous sulfur trioxide exhibits an electrical dipole moment of 0, stemming from the 120-degree angles formed by the S-O bonds.
Physical Properties of Sulfur Trioxide
Sulfur trioxide (SO3) can be comprehensively characterized by a set of physical and chemical properties, each of which offers valuable insights into its nature and behavior:
Chemical Formula: The chemical formula of sulfur trioxide is succinctly denoted as SO3, providing a clear and concise representation of its elemental composition.
Density: Sulfur trioxide exhibits a density of 1.92 grams per cubic centimeter (g/cm3), indicating the mass of the substance per unit volume. This density figure reflects its compactness and relative weightiness.
Molecular Mass: The molecular mass of sulfur trioxide is precisely calculated at 80.066 grams per mole (g/mol). This parameter denotes the mass of one mole of the compound, aiding in its quantitative analysis and formulation.
Boiling Point: Sulfur trioxide undergoes a phase change at a relatively low temperature, with a boiling point of 44.9 degrees Celsius (44.9 °C). This property is significant in various industrial applications where temperature control is crucial.
Melting Point: The melting point of sulfur trioxide is observed at 16.9 degrees Celsius (16.9 °C). This temperature represents the point at which the solid form of the compound transitions into its liquid state.
Odor: Notably, sulfur trioxide is characterized as odorless, lacking any discernible scent or aroma. This property is significant as it distinguishes it from substances with distinctive odors.
Appearance: In terms of its visual characteristics, sulfur trioxide presents itself as a colorless to white crystalline solid. Its appearance as a solid crystalline substance is indicative of its molecular arrangement in a structured lattice.
Complexity: The complexity of sulfur trioxide is quantified at 61.8, a measure that relates to its intricate molecular structure and the various intermolecular forces at play within the compound.
Hydrogen Bond Acceptor: Sulfur trioxide possesses the capacity to accept hydrogen bonds, with three hydrogen bond acceptor sites within its molecular structure. This property underscores its ability to engage in hydrogen bonding interactions with other molecules.
Solubility: Sulfur trioxide exhibits solubility in water, indicating its capacity to dissolve and form a homogeneous solution when placed in an aqueous environment. This solubility property can have implications in various chemical processes and reactions involving the compound.
Also Check – Bond Order Formula
Chemical Properties of Sulfur Trioxide
Sulfuric acid is produced when sulfur trioxide reacts with water:
SO 3 + H 2 O → H 2 SO 4
Interaction of sulfur trioxide with sodium hydroxide yields sodium hydrogen phosphate:
SO 3 + NaOH → NaHSO 4
Also Check – Charles Law Formula
Uses of Sulfur Trioxide
Sulfur trioxide finds application in various industrial and commercial processes:
- It serves as a bleaching agent to eliminate residual hydrogen peroxide and as a digestion agent for separating pulp from lignin.
- In the oxidation of sulfur dioxide to sulfur trioxide, it acts as a catalyst.
- It is employed as a powerful inorganic acid mist, based on sulfuric acid, in industrial and commercial product manufacturing.
- Sulfur trioxide is a crucial reagent in sulfonation reactions.
- This substance is utilized in the production of photovoltaic cells and solar energy devices.
sulfur trioxide (SO3) emerges as a chemically versatile and industrially indispensable compound. Its significance is rooted in its diverse range of applications across multiple sectors. It serves as a dual-purpose agent in the paper industry, aiding in both bleaching processes and the separation of pulp from lignin. Furthermore, SO3 acts as a catalyst in the oxidation of sulfur dioxide, a vital step in the production of sulfuric acid.
In the manufacturing, sulfur trioxide takes on a role as a potent inorganic acid mist, contributing to various chemical reactions and product formulations. Its involvement as a crucial reagent in sulfonation reactions is fundamental in the synthesis of essential compounds used in detergents, dyes, and pharmaceuticals.
Beyond its industrial applications, sulfur trioxide also plays a part in advancing sustainable energy solutions. Its integration into the production of photovoltaic cells and solar energy devices contributes to the harnessing of solar power for electricity generation, aligning with global efforts to transition towards renewable energy sources.
Sulfur trioxide's multifaceted nature and adaptability underscore its significance in modern industrial processes and its role in promoting sustainable and environmentally friendly technologies.
Also Check – Combined Gas Law Formula
Sulfur Trioxide Formula FAQs
What is sulfur trioxide (SO3)?
What is the density of sulfur trioxide?
What is the molecular mass of SO3?
At what temperature does sulfur trioxide boil?
What is the melting point of sulfur trioxide?