
It is defined as a chemical compound with the formula H 2 SO 3 , Sulfurous Acid is also known as Sulfur dioxide solution or trioxosulfuric acid. Acting as an intermediate species that contributes to acid rain, it is identifiable by its pungent odor of burning sulfur and corrosive properties towards metals and tissue. As a sulfur oxoacid and the conjugate acid of hydrogen sulfite, it can also be described as a colorless liquid and a tautomer of sulfonic acid.
Molecular Formula of Sulfuric Acid
The acid's chemical structure shows that the sulfur atom is in the center, and three oxygen atoms are connected by a single bond and a double bond to form the molecule. A single bond connects the two hydrogen atoms to the oxygen atoms. The sulfurous acid structural formula is normally written as:
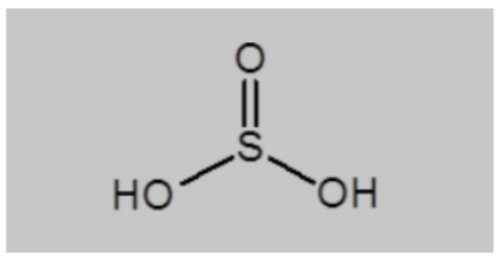
Sulfurous Acid Chemical Formula
Sulphurous acid has the formula H 2 SO 3 , with two hydrogen atoms, three oxygen atoms, and one sulfur atom in the molecule.
| Formula | H 2 SO 3 |
| Molar Mass | 82.07 g/mol |
| Density | 1.03 g/mL |
| Boiling Point | -60 °C |
Properties of Sulfurous Acid – H 2 SO 3
| H 2 SO 3 | Sulfurous acid |
| The molecular weight of H2SO3 | 82.07 g/mol |
| No. of hydrogen bond acceptor | 4 |
| Monoisotopic mass of Sulfurous Acid | 81.97 g/mol |
| No. of hydrogen bond donor | 2 |
| Sulfurous Acid Vapor Pressure | Its vapor pressure is 1740 kPa at about 21 °C. |
| Sulfurous Acid Acidity | On the pH scale, sulphurous acid has a pH of 1.5. It's not a very weak acid, but it's also not a particularly powerful acid. |
Also Check – Atomic Mass Formula
Sulfurous Acid - A Reducing Agent or Oxidizing Agent
Sulfurous acid, or sulfurous acid, has the chemical formula H 2 SO 3 . While no evidence exists in a solution, it has been detected in its gaseous form. The conjugate bases of this elusive acid are common anions such as bisulfite (or hydrogen sulfite) and sulfite. A weak and unstable acid is formed when sulfur dioxide dissolves in water. The compound's presence in a solution cannot be fully explained but has been observed in its gaseous phase. In addition to being a reducing agent, it also acts as a bleaching agent. Due to its only forming in aqueous solutions, it cannot be isolated in its pure state.
Reasons Behind Some of the Acids being Oxidizing and Some Non-oxidizing
The Bronsted Lowry acids, known as proton donors, are oxidizing agents because they reduce H+ to H2 gas. However, oxidizing solid acids, such as sulphuric acid (H 2 SO 4 ), nitric acid (HNO 3 ), chromic acid (H 2 CrO 4 or H 2 Cr 2 O 7 ), chloric acid (HClO 3 ) and perchloric acid (HClO 4 ), contain anionic forms of oxygen. In contrast, phosphoric acid is a weak oxidizing acid due to its low affinity for oxygen compounds.
Also Check – Elevation of Boiling Point Formula
Reactions of Sulfurous Acid
According to SO 2 Raman spectra solution, it represents that the signal intensities are consistent with the equilibrium given as below: SO 2 + H 2 O ⇌ HSO − 3 + H + where pK a = 1.81 and K a = 1.54×10 −2 .
H 2 SO 3 Uses (Sulfurous Acid)
In industry, sulfurous acid is used as a reducing agent, intermediate, and disinfectant. It is also used in the manufacture of paper products.
Also Read – Malic Acid Formula
Health Hazards
Ingesting or inhaling sulfurous acid solution or skin contact causes a severe injury that can result in death. It is corrosive, non-combustible, and toxic. In its molten state, sulfurous acid can cause severe eye and skin burns. As a result, skin contact with this compound is not recommended. As well as releasing toxic, irritating, and corrosive gases, sulfurous acid also causes various health effects.
Chronic Health Effects
Chronic (long-term) health effects may occur months or years after exposure to sulfurous acid:
According to the New Jersey Department of Health & Senior Services, sulfurous acid has not been tested for its ability to cause cancer in animals.
Sulfurous acid compound has not been tested for its reproductive effects.
Sulfurous acid can also irritate the lungs, causing bronchitis with phlegm, cough, and/or shortness of breath due to repeated exposure.
Sulfurous Acid Formula FAQs
Q1. What is the formula for sulfurous acid?
Q2. How is sulfurous acid formed?
Q3. What are the properties of sulfurous acid?
Q4. What are the uses of sulfurous acid?
Q5. Is sulfurous acid dangerous?










