
CBSE Worksheet for chapter-1 Chemical Reactions And Equation class 10
Worksheet For class 10
This page is prepared by the Academic team of Physics Wallah which consists of CBSE Board Worksheet for Class 10 Chemistry . Students of Class 10 Chemistry can get a free Worksheet for Class 10 Chemistry in PDF format prepared as per the newest syllabus and examination pattern in your schools.
Standard 10 students can practice questions and answers which are given here for Chemistry in Grade 10 that will help them to improve their knowledge of all important chapters and their topics. Students can also download free pdf of Class 10 Chemistry Notes prepared by teachers and solve important problems provided here with solutions on daily basis to get more scores in school exams and tests.
Summary
- Introduction of chemical reactions and characteristics of chemical reactions.
- Balanced and unbalanced equations and balancing of equations.
- Information obtained from chemical equations.
- Limitations of chemical equations and their specifications.
Types of Chemical Equations-
- Combination(synthesis)
- Decomposition(analysis)
- Displacement
- Double decomposition(displacement)
- Oxidation and reduction.
- Effect of oxidation in our daily life- combustion, respiration, pollution, corrosion and rancidity.
Section - 1
Q1. All combustion reactions are –
- Endothermic
- Exothermic
- both (A) & (B)
- None of these
Q2. In which of the following reactions, colour change is occurring ?
-
CuCO₃
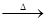 CuO + CO₂
CuO + CO₂
-
CaCO₃ + 2HCl
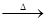 CaCl₂+ H₂O+ CO₂
CaCl₂+ H₂O+ CO₂
- Both (A) and (B)
-
2NaHCO₃
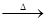 Na₂CO₃+H₂O+CO₂
Na₂CO₃+H₂O+CO₂
Q3. In the equation, aAl+bH₂SO₄
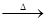 cAl₂(SO₄)
3
+dH₂
cAl₂(SO₄)
3
+dH₂
a, b, c, d are respectively
- 2, 3, 1, 1
- 2, 3, 1, 3
- 2, 3, 2, 3
- 2, 2, 3, 3
Q4. Which of the following reactions is/are also called partner exchange reaction ?
- Neutralization reaction
- Precipitation reaction
- Both (A) & (B)
- Chemical decomposition
Q5. In which of the following reactions, a white precipitate is formed ?
-
AgNO₃(aq) +NaCl(aq)
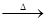 AgCl(s) +NaNO₃(aq)
AgCl(s) +NaNO₃(aq)
-
Pb(NO₃)₂(aq) +Na₂SO₄(aq)
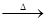 PbSO₄(s) + 2NaNO₃(aq)
PbSO₄(s) + 2NaNO₃(aq)
- both (A) and (B)
- None of these
Q6. Which of the following is a redox reaction ?
- H₂+ Br₂→ 2HBr
-
2NaCl + H₂SO₄
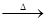 Na₂SO₄ + 2HCl
Na₂SO₄ + 2HCl
-
HCl +AgNO₃
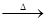 AgCl + HNO₃
AgCl + HNO₃
-
NaOH + HCl
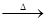 NaCl + H₂O
NaCl + H₂O
Q7. In reaction,
2Ag + 2H₂SO₄ Ag₂SO₄ + 2H₂O + SO₂
H 2 SO 4 acts as a –
- Reducing agent
- Oxidizing agent
- Dehydrating agent
- None
Section - 2
Q8. Indicate the substance oxidized, reduced, acting as reducing agent &oxidizing agent in the following reactions.
- Cl₂ + 2KI 2KCl + I₂
-
MgO + C
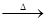 Mg + CO
Mg + CO
-
Fe + 2HCl
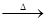 FeCl₂ + H₂
FeCl₂ + H₂
Q9. Mention the type of reaction in each of the following-
- CaCO 3 + H₂O+CO 2 Ca(HCO 3 ) 2
- CH 4 + O 2 CO 2 + 2H 2 O
- Pb + CuCl₂ PbCl 2 + Cu
- Combination reaction (B) Redox reaction (C)Displacement
Q10. Give a balanced chemical equation for each of the following –
- a combination of two compounds
- reaction involved in white washing of walls.
- displacement of a less reactive non-metal by amore reactive non-metal.
Q11. Complete and balance the following reaction-
-
Pb(NO₃)₂
-
(NH₄)₂Cr₂O₇(s)
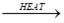
- NH₃+CuO
Q12. What happens when CO₂(g) is bubbled through lime water
- in small amount?
- in excess?
Q13. On mixing the solution of lead nitrate and potassium iodide prepared in water
- write the chemical reaction involved in balanced form
- What is the colour of the precipitate ? Name the precipitate.
Q14. Write the following reactions in the form of chemical equations and balance them.
- Calcium oxide reacts with water to form calcium hydroxide
- Magnesium reacts with nitrogen to form magnesium nitride
- Potassium chlorate decomposes to form oxygen and potassium chloride.
Frequently Asked Question (FAQs)
Q1. Give some questions to ask about Chemical Reactions?
Ans. Chemical Reaction Questions
- What is the difference between physical and chemical processes in chemistry?
- When and How does the breaking of chemical bonds release energy?
- Why is mass conserved in chemical reactions?
Q2. What is the importance of chapter - chemical equation class 10?
Ans. Following are the importance are mentioned below:
- The chemical equation describes briefly the names of the reactants, the products, and the state of matter.
- It explains the specific conditions of temperature, pressure, catalyst, etc., under which the reaction takes place.
- It also states whether energy is evolved or needs to be supplied.
Q3. Write the four types of chemical reactions Class 10?
Ans. The four types of chemical reactions in Class 10 are:
- Combination Reaction
- Decomposition Reaction
- Displacement Reaction
- Precipitation Reaction
- Double Displacement Reaction
Q4. Who discovered chemical equations?
Ans. In 1661, when Boyle defined an element as a substance that can't be decomposed into a simpler substance by a chemical reaction, only 13 elements were known: antimony, arsenic, bismuth, carbon, copper, gold, iron, lead, mercury, silver, sulfur, tin, and zinc.
Q5. Who discovered chemical equations?
Ans. In 1661, when Boyle defined an element as a substance that can't be decomposed into a simpler substance by a chemical reaction, only 13 elements were known: antimony, arsenic, bismuth, carbon, copper, gold, iron, lead, mercury, silver, sulfur, tin, and zinc.









