
CBSE Class 11 Chemistry Notes Chapter 3: In ch 3 chemistry class 11 notes, we learn about how elements are grouped and organized based on their properties.
This organization helps us understand the behavior of different elements better. We study the periodic table, which is like a big chart showing all the elements arranged in a specific order.
This order is based on things like the number of protons in an atom and how the electrons are arranged around the nucleus. By studying this, we can see patterns in how elements behave.CBSE Chemistry Notes Class 11 Chapter 3 Classification of Elements and Periodicity in Properties
Dobereiner's Triads
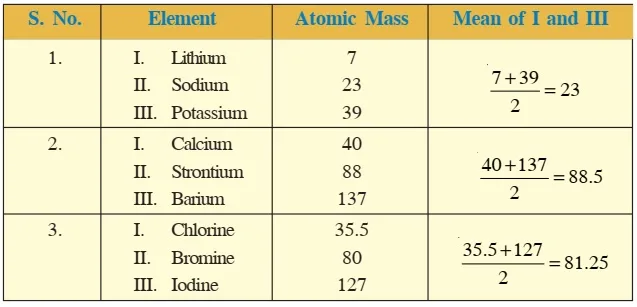 Dobereiner's Triads were proposed by the German chemist Johann Wolfgang Dobereiner. He attempted to classify elements with similar properties into groups of three elements each, called "triads.
Dobereiner's Triads were proposed by the German chemist Johann Wolfgang Dobereiner. He attempted to classify elements with similar properties into groups of three elements each, called "triads.Dobereiner's Triads had limitations:
Not all elements known at that time could be classified into triads. Only four triads were identified by Dobereiner, leaving many elements unaccounted for.Newland’s Octaves
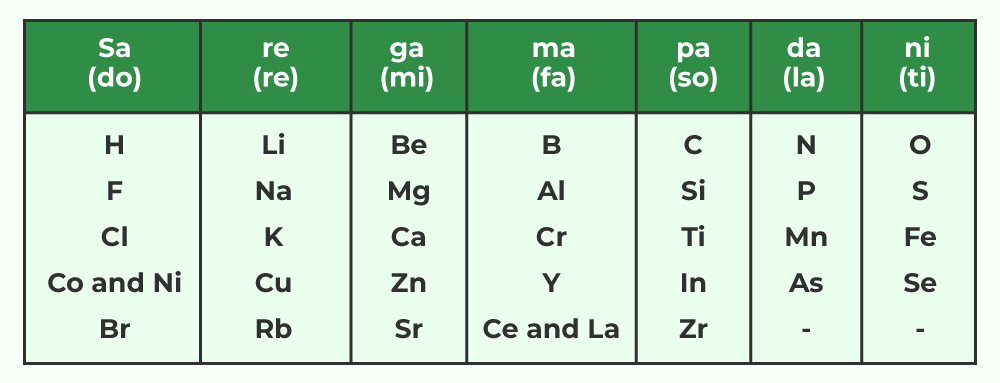 John Newlands, an English scientist, made significant contributions to the early development of the periodic table. In 1866, he arranged the 56 known elements in increasing order of atomic mass. Newlands observed a pattern where every eighth element exhibited properties similar to the first. This observation led to the formulation of Newland’s Law of Octaves. According to this law, when elements are arranged in increasing order of atomic mass, the properties of two elements with an interval of seven elements between them would be similar.
John Newlands, an English scientist, made significant contributions to the early development of the periodic table. In 1866, he arranged the 56 known elements in increasing order of atomic mass. Newlands observed a pattern where every eighth element exhibited properties similar to the first. This observation led to the formulation of Newland’s Law of Octaves. According to this law, when elements are arranged in increasing order of atomic mass, the properties of two elements with an interval of seven elements between them would be similar.
Newland’s octaves had limitations:
The classification of elements based on octaves was only successful up to calcium. The discovery of noble gases posed a challenge to Newland’s arrangement, as they did not fit into the periodic pattern without disrupting it completely.Mendeleev’s Periodic Table
Dmitri Ivanovich Mendeleev, a Russian chemist, introduced his periodic table in 1869. He noticed that the properties of elements, both physical and chemical, repeated periodically with their atomic masses. Mendeleev's Periodic Law, also known as Mendeleev’s Law, states that the chemical properties of elements are a periodic function of their atomic weights.Advantages of Mendeleev’s Periodic table:
It accommodated newly discovered elements without disturbing the table's structure, including germanium, gallium, and scandium. Mendeleev’s table helped correct inaccurate atomic weights that were prevalent at the time. It introduced variations from the strict order of atomic weights.Limitations of Mendeleev’s Periodic table:
Hydrogen's position in the group of alkali metals contradicted its halogen-like qualities. Isotopes were not considered, leading to inconsistencies in the placement of elements like protium, deuterium, and tritium.Modern periodic table
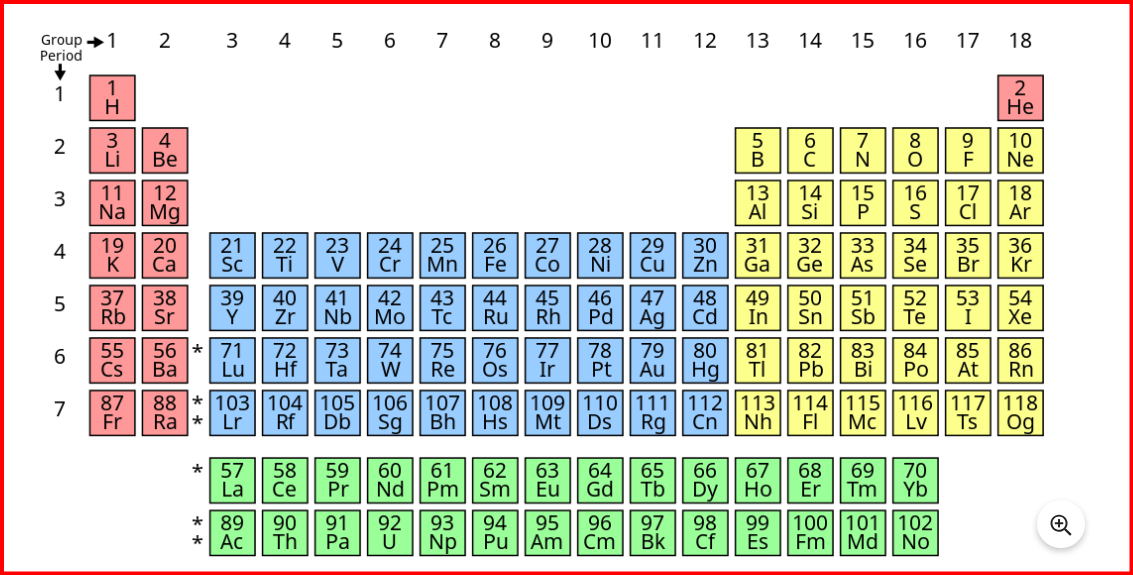 In 1913, English physicist Henry Moseley conducted groundbreaking research on the characteristic x-rays emitted by different metals. He discovered a direct correlation between the square root of the frequency of these x-rays and the atomic number of the elements. Based on his findings, Moseley formulated the modern periodic law, which states:
In 1913, English physicist Henry Moseley conducted groundbreaking research on the characteristic x-rays emitted by different metals. He discovered a direct correlation between the square root of the frequency of these x-rays and the atomic number of the elements. Based on his findings, Moseley formulated the modern periodic law, which states:
"The physical and chemical properties of elements are periodic functions of their atomic numbers."
Unlike atomic mass, which is influenced by the mass of protons and neutrons in the nucleus, the atomic number solely determines the number of electrons in an atom. Since chemical properties primarily depend on the arrangement of electrons in different energy levels, elements with different electronic configurations exhibit distinct chemical behaviors. Therefore, Moseley argued that atomic number, not atomic mass as proposed by Mendeleev, should be the basis for classifying elements in the periodic table. The periodic repetition of similar properties among elements grouped by their atomic numbers is known as periodicity. This concept highlights the predictable patterns in the chemical and physical properties of elements as their atomic numbers increase.Classification of elements in modern periodic table
The modern periodic table comprises 18 vertical columns known as groups (1-18) and 7 horizontal rows known as periods. Each period and group represent distinct characteristics of the elements.- The first period consists of only two elements: Hydrogen and Helium.
- The second period includes eight elements, starting from Lithium and ending with Neon.
- The third period also contains eight elements, from Sodium to Argon.
- The fourth period encompasses eighteen elements, extending from Potassium to Krypton.
- The fifth period also holds eighteen elements, ranging from Rubidium to Xenon.
- The sixth period consists of thirty-two elements.
- The seventh period is incomplete.
- The elements in the 1st and 2nd groups are termed s-block elements, with a general electronic configuration of ns1-2.
- Elements in groups 13th to 18th are known as p-block elements, having a general electronic configuration of ns2 np1-6.
- The elements from the 3rd to 12th groups belong to the d-block, with a general electronic configuration of (n-1)d1-10 ns1-2.
- Lanthanides and actinides, placed separately at the bottom of the periodic table, are known as f-block elements, with a general electronic configuration of (n-2)f1-14 (n-1)d0-1 ns2.
Periodic properties and their trends
The periodic properties of elements are closely related to their electronic configuration and exhibit a gradual change as we move down a group or across a period. Key physical properties such as melting points, boiling points, density, and enthalpy of fusion and vaporization are influenced by electronic configuration. However, our focus lies primarily on properties like atomic and ionic radii, ionization enthalpy, electron gain enthalpy, and electronegativity.Atomic and Ionic Radii:
Atomic Radii: The distance from the nucleus to the outermost electron shell defines atomic radii, which can be covalent, van der Waals, or metallic.
Covalent radii: Half the distance between nuclei of adjacent atoms in a single covalent bond.
Van der Waals radii: Half the internuclear distance between atoms of neighboring molecules in a solid.
Metallic radii: Half the distance between nuclei of adjacent atoms in a metallic crystal.
Atomic radii decrease across periods due to increased effective nuclear charge and increase down groups owing to additional electron shells and shielding effect.Ionic Radii: The effective distance from the nucleus to the electron cloud for ions formed from neutral atoms.
Ionic radii follow the same trend as atomic radii, decreasing across periods and increasing down groups.Properties of an Element
Atomic radius is a fundamental concept in chemistry that refers to the size of an atom. However, due to the electron's probability distribution, it's challenging to measure the exact size of an isolated atom. Therefore, different forms of atomic radius are utilized depending on the atom's surroundings.Ionization Enthalpy
The energy required to remove an electron from the outermost orbit of an isolated gaseous atom. Increases across periods due to increased nuclear charge and decreases down groups.Electron Gain Enthalpy
The change in enthalpy when a gaseous atom gains an electron to form a monovalent anion. Increases across periods and decreases down groups, with exceptions for half-filled or fully filled orbitals.Electronegativity
- The ability of an atom to attract shared electrons in a covalent bond.
- Fluorine is the most electronegative, while cesium is the least.
- Electronegativity increases across periods and decreases down groups.
Periodic Trends in Chemical Properties
Valence electrons are crucial in determining the chemical behavior of atoms, as they reside in the outermost shell and are involved in bonding. The valence, or valency, of an atom corresponds to the number of these electrons. For s- and p-block elements, the valence is typically equal to the number of valence electrons.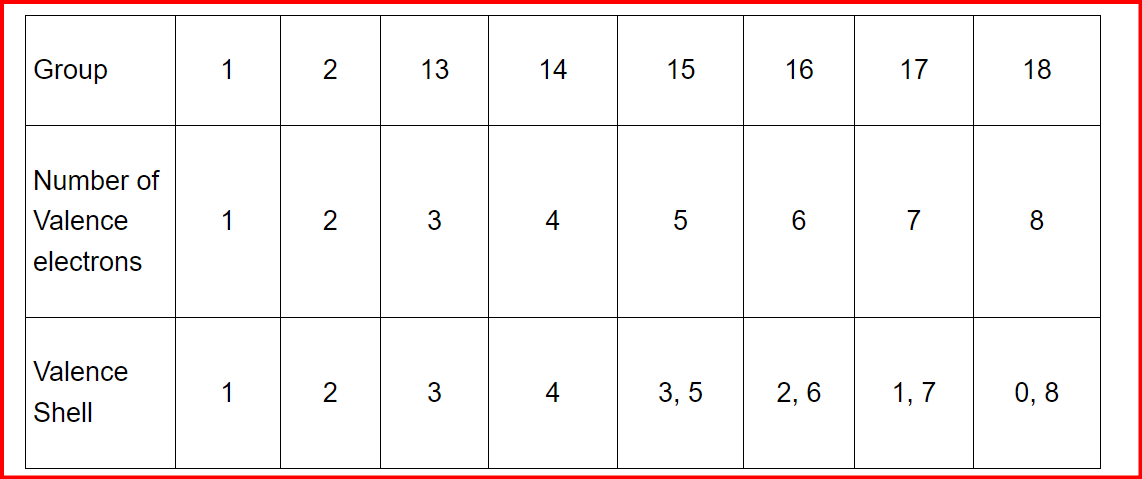 Along a period, the number of valence electrons increases from 1 to 8 as we move from left to right. However, when considering the valence in compounds relative to hydrogen or oxygen, it peaks at 4 before declining to 0. For instance, in Na2O, oxygen, being more electronegative, gains two electrons from each sodium atom, leading to an oxidation state of -2, while sodium loses one electron, resulting in an oxidation state of +1. Within a group, the number of valence electrons remains constant, resulting in elements within the group sharing the same valence. For instance, all alkali metals in group 1 have a valence of one, while alkaline earth metals in group 2 have a valence of two. Noble gases in group 18 have zero valence, as they are chemically inert and do not form bonds.
Along a period, the number of valence electrons increases from 1 to 8 as we move from left to right. However, when considering the valence in compounds relative to hydrogen or oxygen, it peaks at 4 before declining to 0. For instance, in Na2O, oxygen, being more electronegative, gains two electrons from each sodium atom, leading to an oxidation state of -2, while sodium loses one electron, resulting in an oxidation state of +1. Within a group, the number of valence electrons remains constant, resulting in elements within the group sharing the same valence. For instance, all alkali metals in group 1 have a valence of one, while alkaline earth metals in group 2 have a valence of two. Noble gases in group 18 have zero valence, as they are chemically inert and do not form bonds.
Anomalous Properties of Second Period Elements
Diagonal relationships in the periodic table occur when elements from the second period share similarities with elements from the third period when placed diagonally across each other, despite being in different groups.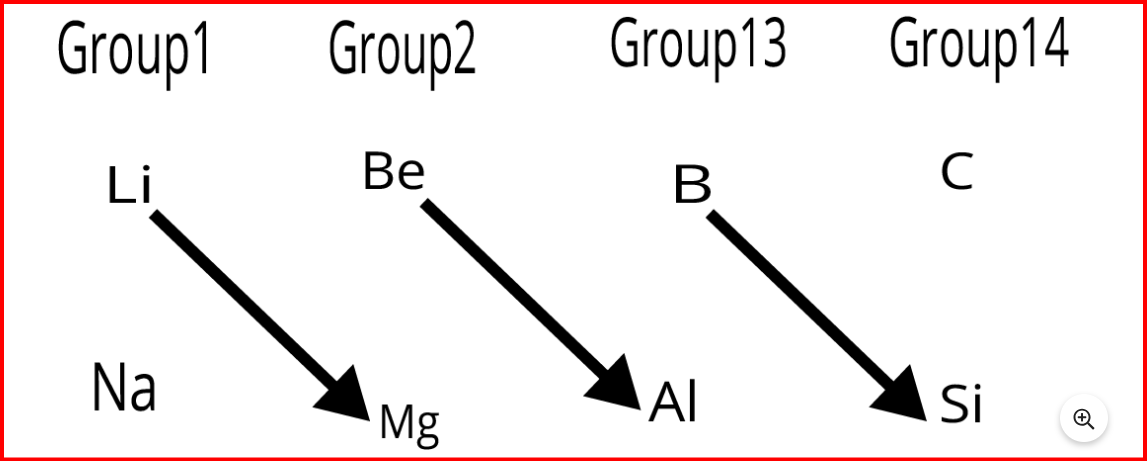 Consequently, the maximum covalency of the first member is limited to 4, while the subsequent members can expand their valence shell to accommodate more than four pairs of electrons.
Consequently, the maximum covalency of the first member is limited to 4, while the subsequent members can expand their valence shell to accommodate more than four pairs of electrons.Periodic Trends and Chemical Reactivity
CBSE Class 11 Chemistry Notes Chapter 3
Study without using the internet
Benefits of CBSE Class 11 Chemistry Notes Chapter 3
Concept Clarity: These notes provide a structured overview of the classification of elements and periodic trends, helping students understand fundamental concepts clearly.
Comprehensive Coverage: The notes cover all essential topics and subtopics of the chapter, ensuring that students have a thorough understanding of the subject matter.
Simplified Explanation: Complex concepts are explained in a simplified manner, making it easier for students to grasp and retain information.
Quick Revision: Students can use these notes for quick revision before exams or assessments, helping them consolidate their knowledge effectively.
Practice Questions: The notes include practice questions and examples that enable students to test their understanding and reinforce their learning.
Exam Preparation: By studying these notes, students can also practice Important Questions for Class 11 Chemistry for their exams, as they cover the entire syllabus prescribed by the CBSE board.
CBSE Class 11 Chemistry Notes Chapter 3 FAQs
What is the Periodic Law?
What are the different blocks in the periodic table based on electronic configuration?
How does atomic radius vary across a period and down a group?
Explain electron gain enthalpy and its periodic trends.
What is electronegativity? How does it vary in the periodic table?










