
CBSE Class 11 Chemistry Notes Chapter 6: In essence, thermodynamics is the study of energy flow. The premise that energy can be transferred and transformed from one form to another is the basis of the entire chapter. The CBSE Class 11 Chemistry Notes Chapter 6 Thermodynamics start by going over the many characteristics of a system because measuring energy requires knowledge of a specific system.
The topic of the thermodynamics chapter in CBSE Class 11 Chemistry is fairly extensive. This chapter covers a variety of subjects, in addition to the necessary mathematical sums. The essential ideas of the chapter must be understood from the beginning. On our official website, students can get the notes in PDF format for free while they prepare for this chapter.Thermodynamics Class 11 Notes Overview
CBSE Class 11 Chemistry Notes Chapter 6 Thermodynamics the study of energy transformations. It introduces key concepts such as the laws of thermodynamics, internal energy, enthalpy, and entropy. The chapter explains the first law of thermodynamics, emphasizing energy conservation in physical and chemical processes. It also discusses exothermic and endothermic reactions, heat capacity, and the significance of enthalpy changes. Additionally, the chapter covers the second law of thermodynamics, introducing entropy as a measure of disorder and spontaneity in reactions. Overall, it lays the foundation for understanding energy changes in chemical systems.CBSE Class 11 Chemistry Notes Chapter 6 PDF
The study of the relationships between work, temperature, heat, energy, radiation, and the physical characteristics of matter is known as thermodynamics. The mechanisms of energy transference and change from one form to another are covered in Chemistry Class 11 Chapter 6. Here we have provided thermodynamics class 11 notes pdf for the ease of the students.CBSE Class 11 Chemistry Notes Chapter 6 PDF
CBSE Class 11 Chemistry Notes Chapter 6
Thermodynamics is the study of the movement of mass, heat, and energy.Thermodynamics terminology
-
System:
-
Surrounding:
The remaining part of the universe except for the system which isn’t kept under observation is known as surroundings.
In general, it can be stated as;
Universe = System + Surrounding
Types of the system
a) Open system –
The system where the flow of both, mass and heat energy takes place.
Example: Human body.
b) Closed system –
The system where the flow of heat energy takes place but has constant mass.
Example: Pressure cooker.
c) Isolated system –
The system where none of the flow takes place.
Example: Thermos flask.
State of the system
It is possible to define and modify the system's state about modifications in the state variables, P, V, T, and n. Any change in one of these variables will alter the system's status because they determine its circumstances.Properties of the system
-
Intensive properties –
Properties are mass- and number-independent, depending only on the concentration of the particles in the system. These include density, refractive index, pressure, and so on.
-
Extensive properties –
Thermodynamic equilibrium
When the three types of equilibrium listed below are satisfied and the state variables remain unchanged, the system remains in equilibrium.-
Mechanical equilibrium –
The absence of mechanical motion, constant pressure, and volume brings up the mechanical equilibrium.
-
Thermal equilibrium –
The constant heat and temperature concerning time bring up thermal equilibrium.
-
Chemical equilibrium –
The rate of forward reaction equal to the rate of backward reaction brings up the chemical equilibrium.
Internal energy
Internal energy, frequently represented by the letters U or E, is the total of the energy's constituent parts that are impacted by the system's internal elements. The system under observation acts as an ideal gas system that depends only upon kinetic energy and hence, is the function of temperature as U ∝ T . Thus, the internal energy is a state function.Modes of energy transport
Heat
Heat (Q) is the energy that is transmitted as a result of temperature differences in the system and its surroundings. The molecules' kinetic energy increases with system temperature, raising the internal energy as a result.Work:
Work (W) is the amount of energy used to overcome the external forces operating upon the system. A system's internal energy decreases as it expands. On the other hand, internal energy increases as the system contracts.The first law of thermodynamics:
The first law of thermodynamics states that energy can neither be created nor destroyed.
The sign conventions are given as;
Work done by the system = - W
Work done on the system = + W
Heat flows into the system = + Q
Heat flows out of the system = - Q
Reversibility
The process can retrace its beginning course and arrive at the same initial state by making a very tiny, or infinitesimal, modification in the system or surroundings. There must be no dissipative forces and the system must be in a quasi-static state for a process to follow reversibility.-
Quasi-static state –
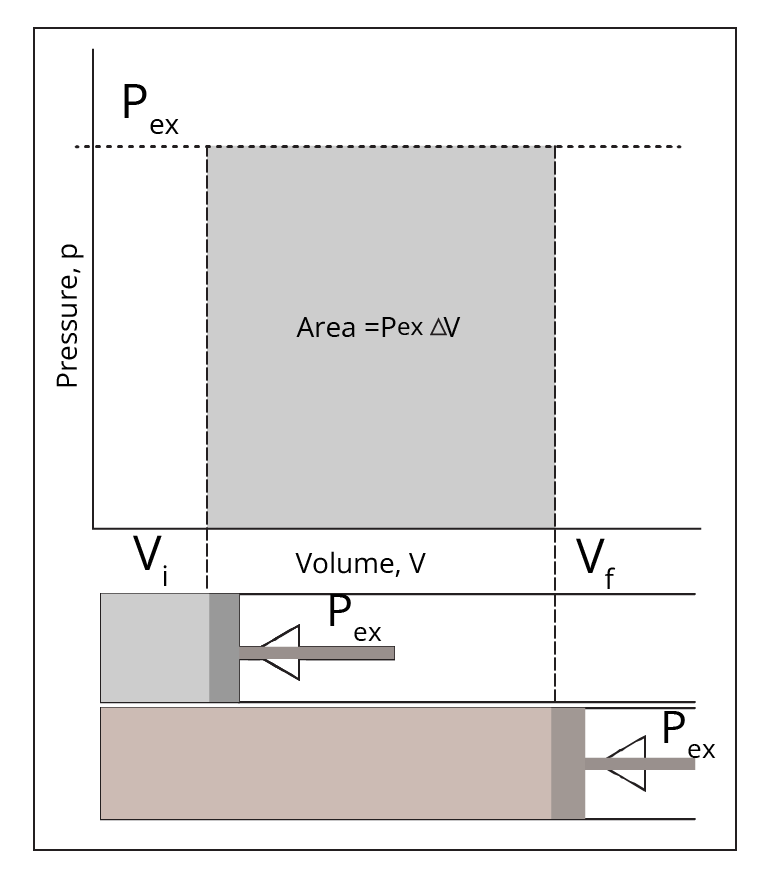
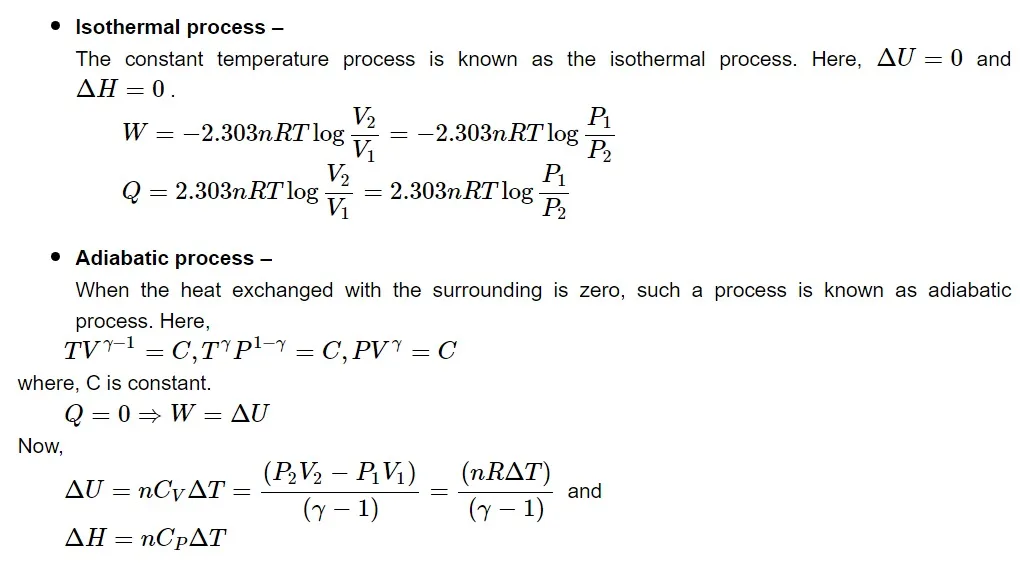
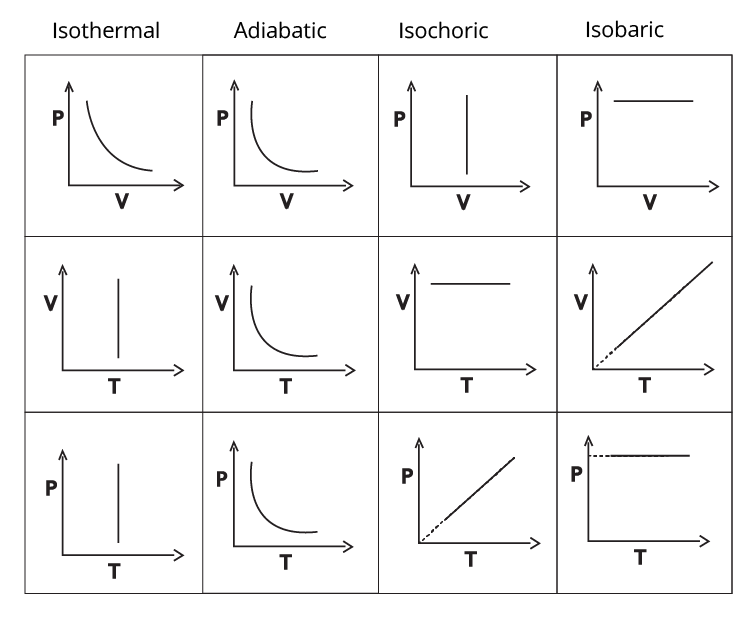
Types of thermodynamic processes
Benefits of CBSE Class 11 Chemistry Notes Chapter 6
The ability of rapid revision notes to assist students in breaking down a great deal of material into accessible portions is one of its main advantages. Students studying chemistry frequently have a lot of formulas, equations, and theories to understand. Although these can be challenging to recall and comprehend, students can quickly and readily access this material when they need it with the use of short revision notes. Students can concentrate on what is most important and save time by summarising a topic's primary ideas rather than attempting to remember unimportant information.CBSE Class 11 Chemistry Notes Chapter 6 FAQs
Which is the most important chapter in chemistry class 11?
Is Class 11 chemistry difficult?
Which is the most difficult chapter in class 11 chemistry?










