
CBSE Class 11 Chemistry Notes Chapter 8: Understanding the traditional notion of Redox Reactions—which covers oxidation and reduction reactions in addition to other topics like electrode processes, oxidation number, and electron transfer reactions—is the main goal of CBSE Class 11 Chemistry Notes Chapter 8.
With the help of these notes, students can rewrite the entire chapter and simultaneously learn about all of the main ideas. Additionally, students can use this to study more efficiently, stay ahead in class, and do well on tests.CBSE Class 11 Chemistry Notes Chapter 8 PDF
These redox reaction class 11 notes are conveniently available to assist students study for the exams and make the material easier to understand. Students can take notes and get instantly familiar with all the important concepts by consulting these notes.CBSE Class 11 Chemistry Notes Chapter 8 PDF
CBSE Class 11 Chemistry Notes Chapter 8
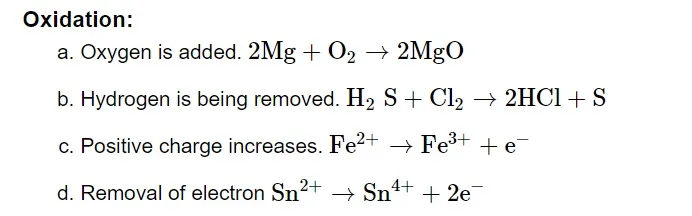
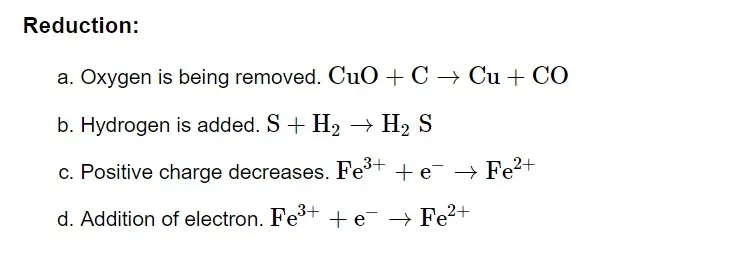
Oxidation Number
An element acquires an imaginary or appearing charge over each atom when it changes from its elemental free state to its combined form in molecules. It is ascertained by applying a random set of guidelines. In a specific bonded state, the charge is relative. It has proven possible to use oxidation numbers as a more useful method to monitor electron changes in chemical reactions involving compound formation. Throughout this process, the full transfer of electrons from a less electronegative to a more electronegative atom is always anticipated.Rules Governing Oxidation Number
The oxidation number of each element in a variety of compounds can be determined using the following formulas. It's crucial to keep in mind that these guidelines are based on the element's electronegativity.Atom of Fluorine
Fluorine is the most electronegative of all the elements (known). In all of its compounds, it has an oxidation number of –1.

Hydrogen Atom
The hydrogen atom has an oxidation number of +1 in general. However, it is –1 in metallic hydrides (e.g. NaH, KH).
Halogen Atom
In general, all halogen atoms (Cl, Br, and I) have an oxidation number of –1.
If a halogen atom is connected to a more electronegative atom than the halogen atom, the oxidation numbers will be positive.
Metals
Alkali metals (Li, Na, K, Rb, etc.) always have an oxidation number of +1. For alkaline earth metals (Be, Mg, Ca, etc.), the oxidation number is always +2. Aluminum's oxidation number is always +3. Keep in mind that a metal's oxidation number could be zero or negative. The oxidation number of an element is always 0 when it is in the free state or allotropic forms. In a molecule, the total oxidation number of all the atoms is zero. An ion's charge is determined by adding the oxidation numbers of each of its constituent atoms. In the modern periodic table, an element with group number n can have an oxidation number between (n – 10) and (n – 18). (However, it mostly applies to p-block elements).Paradox of Fractional Oxidation Number
The fractional oxidation number is the average of the oxidation states of all the atoms of the element under consideration, and the structural characteristics show that the atoms of the element for which the fractional oxidation state is realized are indeed present in separate oxidation states.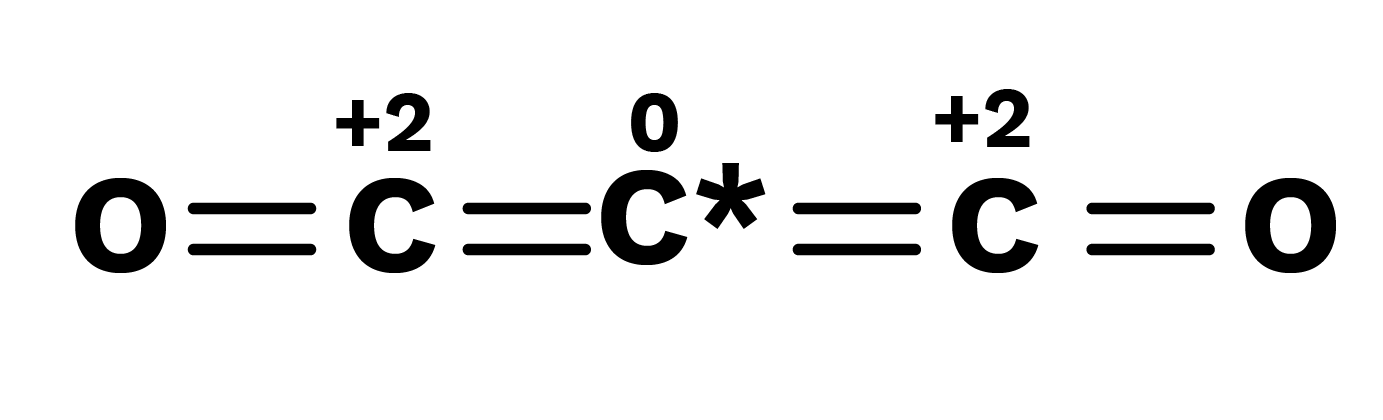
Oxidizing and Reducing Agent
Oxidising Agent or Oxidant
Oxidizing agents are compounds that, in the course of a chemical reaction, can both oxidize and reduce other molecules. Chemicals known as oxidants can change an element's oxidation number in a redox process by adding or subtracting electrons.Reducing Agent or Reductant
Reducing agents are compounds that, in a chemical reaction, can both reduce and oxidise other molecules. Reductants are substances that raise an element's oxidation number or cause it to lose electrons during a redox reaction.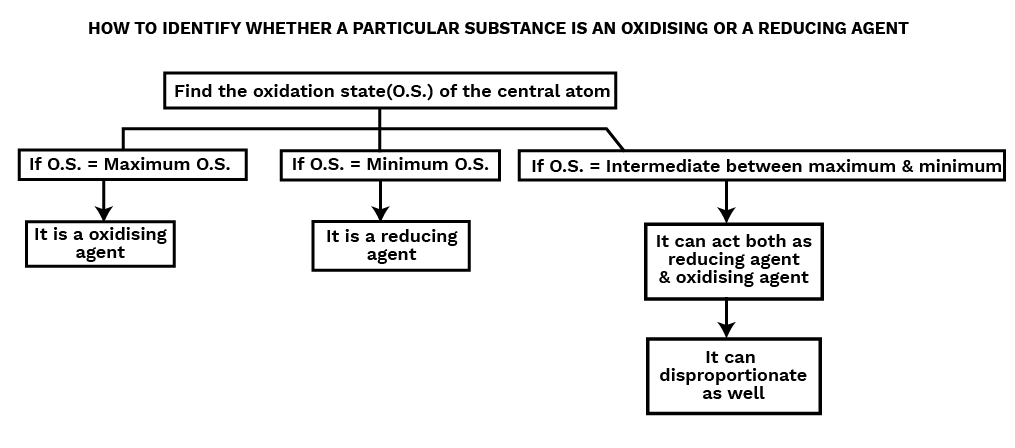
Disproportionation Reaction
A disproportionation reaction is a type of redox reaction in which an element that is present in a particular molecule at a certain oxidation state is simultaneously reduced and oxidised. Disproportionation reactions are a special kind of redox reaction. An element that can exist in at least three oxidation states must always be present in one of the reactants in a disproportionation process.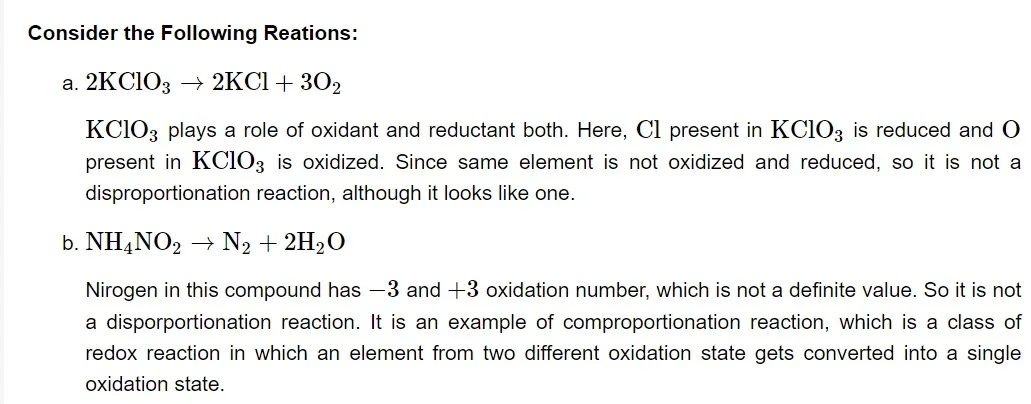
Balancing of Redox Reactions
Two conditions must be met by any balanced equation. Atom balance, also known as mass balance, states that there should be an equal amount of atoms of each species on the reactant and product sides. Charge balance: The total of the real charges on both sides of the equation ought to be the same.The redox equations can be balanced in two ways:
-
Number change technique of oxidation
-
Half-cell method or ion electron method
Because the first approach is not particularly effective in balancing redox reactions, students are urged to use the second way (Ion electron method) to do so.
Acid-Base Reaction
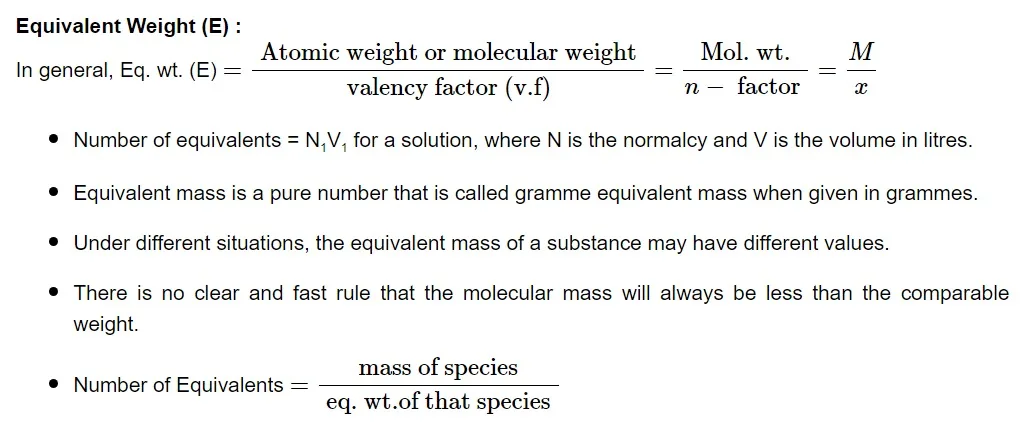
The actual number of hydrogen or hydroxide ions transferred during an acid-base reaction is known as the valence factor. The acid or base may contain more hydrogen or hydroxide ions that can be replaced than what is replaced throughout the reaction. V. f is the number of hydrogen ions from the acid that are substituted for each base molecule.Normality
The normalcy of a solution is defined as the number of equivalents of solute in one liter (1000 mL). Allow W g of the equivalent weight E solute to dissolve in water to produce V mL of solution.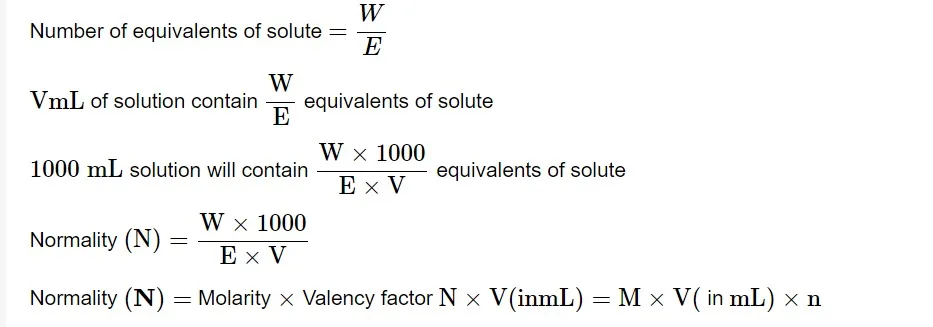
Law of Equivalence
The law states that one equivalent of one element combines with one equivalent of the other. A chemical reaction produces the same number of equivalents or milli equivalents of products separately from equivalents and milliequivalents of reactants in the same proportion.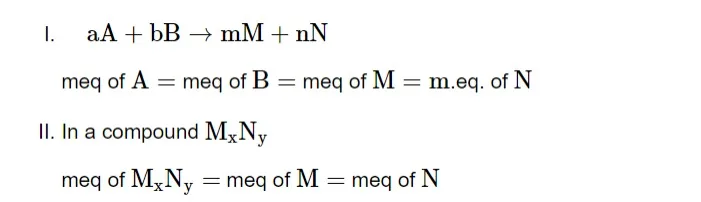
Titrations
By allowing a precisely measured volume to react with a reference solution of a different substance that has a known concentration, titration is a technique for measuring the concentration of a solution. A known concentration solution taken in a burette is called a standard solution. Another name for it is titirant.There are Two Types of Titrants
Primary Titrants/Standards - These reagents can be precisely weighed, and their solutions do not require standardisation before use. Example: oxalic acid, ferrous ammonium sulfate.
Secondary Titrants/Standards: Because these reagents cannot be precisely weighed, their solutions must be standardised before use. Example: NaOH,KOH NaOH,KOH
Titrate: A solution containing the chemical to be measured, usually in a beaker.
The equivalency point is the point at which the number of titrant equivalents added equals the number of titrate equivalents.
Indicators
extra material supplied to enable the titration completion at the equivalency point to be physically detected. Usually, the hue changes when the titration is complete. There are numerous sizes and shapes for titrations.Types of Indicators:
-
Titrations of acid and base (to be studied in Ionic equilibrium)
-
Permanganate titration:
Benefits of Redox Reaction Class 11 Notes
One of the key benefits of quick revision notes is their capacity to help students divide a large amount of material into manageable chunks. There are often a lot of formulas, equations, and theories for students studying chemistry to comprehend. Short revision notes allow students to quickly and easily retrieve this material when needed, even though it can be difficult to remember and understand. By summarising a topic's main principles, students can focus on what matters most and save time instead of trying to recall irrelevant details.CBSE Class 11 Chemistry Notes Chapter 8 FAQs
What are the main points of redox reactions?
What are the essential conditions for a redox reaction?
What are the 4 types of redox reactions?










