
Introduction
Matter is our surrounding of Class 9
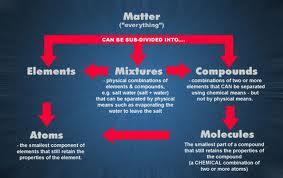
Everything in this universe is made up of material which has been named ‘matter’ by scientists. The air we breathe, the food we eat, the water we drink, stones, clouds, stars, plants and animals or a particle of sand – everything around us is matter.
Since early times, human beings have been trying to understand their surroundings. Early Indian philosophers classified matter in the form of five basic elements – the “Panch Tatva” – air, earth, fire, sky and water. According to them, everything living or non-living, is made up of these five basic elements.
INTRODUCTION
There are a large number of things around us which we see and feel. For example, we can see a book in front of us. A book occupies some space. The space occupied by the book is called it volume. If we pick up the book, we can also feel its weight. So, we conclude that the book has some mass. We cannot see the air around us, yet if we fill a balloon with air and then weight it carefully, we will find that not only does air occupy space (bounded by the balloon), but is also has mass.
Things like a book and air are examples of matter. Other examples of matter are wood, cloth, paper, ice, steel, water, oil etc. Further, that matter offers resistance is borne out by the fact that we cannot displace an object from one place to another without applying some force. We have to apply force to pick up a stone from the ground. Thus, matter can be defined as follows:
“Anything that occupies space, has mass and offer resistance is called matter.”
Substance: A substance is a kind of matter that cannot be separated into other kinds of matter by any physical process. For example, sugar dissolved in water can be separated from water by simply evaporating the water. Here sugar is a substance which cannot be broken into its components by any physical process.
Plasma: The fourth state of matter, It is a hot ionized gas consisting of equal number of +ve and –ve charged ions and electrons because it is made up of electrically charged particle so influence by electric and magnetic fields.
PHYSICAL NATURE OF MATTER:
Matter exists in three physical state Solid, liquid and gas. In Solid the molecule are closely packed, In Liquids lossely packed where as in Gas there is no force of attraction between molecules
Particle Nature of Matter – Matter is made up of particles:
To show the particle nature of matter, we perform the following experiment:
Experiment: Take about 50ml water in a graduated cylinder and dissolve small amount of common salt (NaCl) or sugar in it with the help of a glass rod.
Observation and explanation: The salt or sugar dissolves in water and there is no noticeable change in the level of water. This is because, there are some spaces in between the particles of water, which are occupied by salt or sugar particles (when salt or sugar dissolves in water) and thus the level of water does not rise.
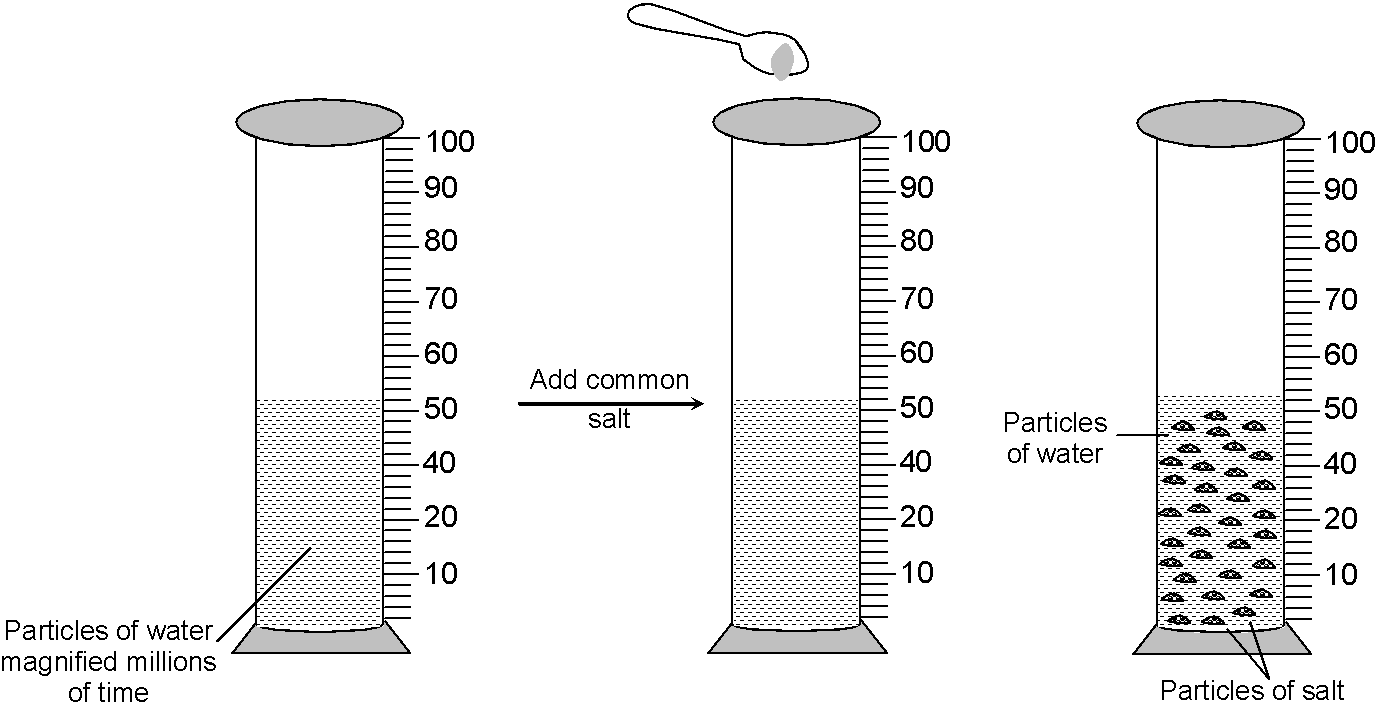
When salt dissolves in water, the particles of salt get into the spaces between the particles of water and the level of solution does not rise
Conclusion : From above experiment, we led to conclude that, there are some spaces between the particles of matter, or in other words matter is made up of particles.
How small are these particles of matter?
To know how small are the particles of matter from which they are made up of, let us perform the experiment:
Experiment: Take about one crystal of potassium permanganate (KMnO 4 ) and dissolve it in 100ml of water. The colour of solution will be dark pink. Take out approximate 10ml of this solution (dark pink) and put it into 90 ml of clear water. Now take 10ml of this solution and put it into another 90ml of clear water. Keep diluting the solution like this 5 – 8 times.
Observation and Explanation: The pink colour will not disappear altogether, though it becomes lighter and lighter with each dilution. This is because, there must be millions of tiny particles present in one crystal of KMnO 4 which keep on dividing into smaller and smaller number with each dilution, thereby making colour lighter and lighter.
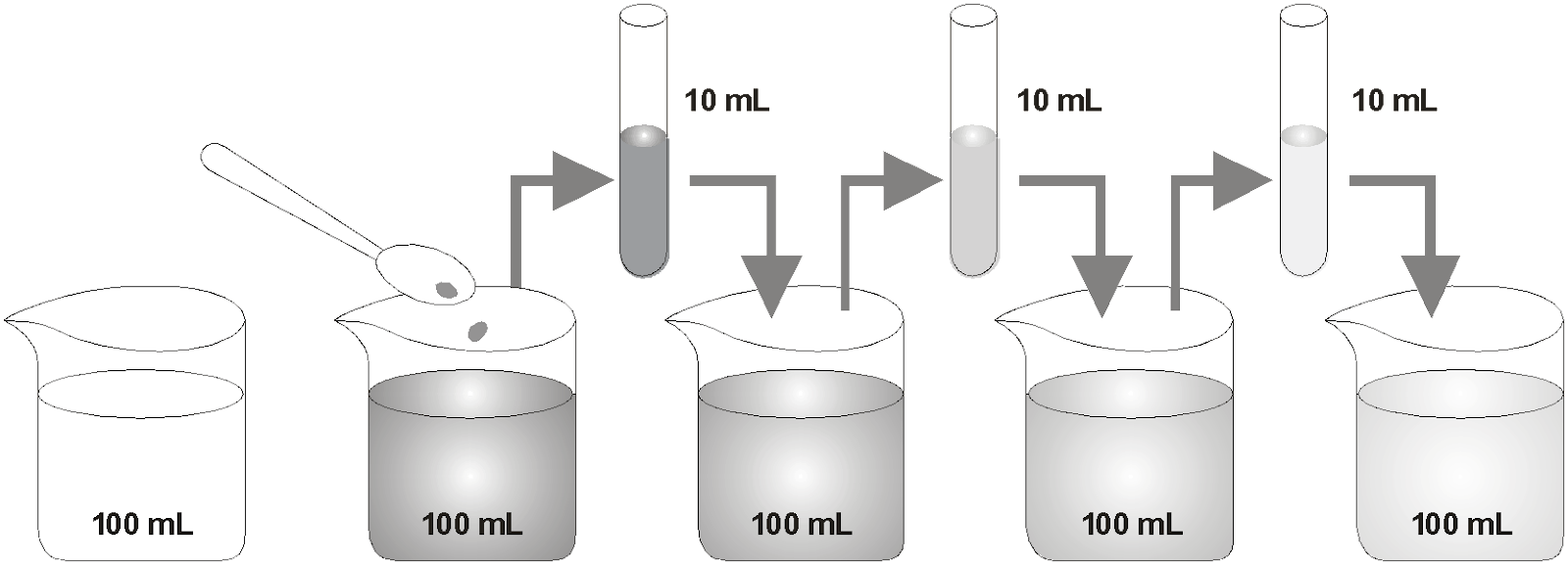
Estimating how small are the particles of matter. With every dilution, though the colour becomes light, it is still visible.
Conclusion : From above experiment we conclude that “matter is made up of extremely small particles which can not be seen even with a powerful microscope.
Characteristics of Particles of Matter :
The important characteristics of particles of matter are the following :
(i) The particles of matter are very, very small : It can be explained by performing the following experiment. We will dissolve 2 or 3 drops of indigo dye in 100 ml of water. We will get a deep blue coloured solution. Now we will keep diluting the solution and we will observe that intensity of blue colour of indigo dye solution goes on decreasing.
(ii) The particles of matter have spaces between them :
(A)Experiment : We take about 100 ml of a water in a beaker and mark the level of water. We will also take 50 g of sugar. Now we will dissolve the sugar by stirring and we get 3 sugar solution.
(B) Conclusion : The level of sugar solution in the beaker is at the same mark where water level was initially in the beaker.
It shows that particles sugar go into the spaces between various molecule of water due to which there is no change in the volume. Thus, from this experiment it can be concluded that, the molecules in water are not tightly packed, they have spaces between them.
(iii) The particles of matter are constantly moving : This property can be explained by diffusion.
Diffusion : “Intermixing of particles of two different types of matter on their own is called diffusion.” It is the phenomenon in which the movement of molecules or particles occur from their higher concentration towards their lower concentration.
e.g. : When a perfume bottle is opened in one corner of a room, its fragrance spreads in the whole room quickly. This happens because the particles of perfume move rapidly in all directions and mix with the moving particles of air in the room.
(iv) Particles of matter attract each other : There are some forces of attraction between the particles of matter which bind them together.
Cohesive Force : The force of attraction between the particles of different substances is called adhesive force.
e.g. : If we take a piece of chalk, a cube of ice and an iron nail and beat them with a hammer, chalk will easily break into smaller pieces, but more force will be required to break a cube of ice and iron nail will not break.
Reason: The reason for this is that force of attraction is quite weak in between the chalk particles, but force of attraction in between the particles of ice cube is a bit stronger, while force of attraction in between the particles of iron is very-very strong.
NCERT solutions for class 9 Science prepared by pw will help you to solve your NCERT text book exercise.









