
Classification of Proteins
Molecules of Cell of Class 11
1. According to Structure
Fibrous :
Secondary structure most important (little or no tertiary structure)
Insoluble in water. Physically tough.
Long parallel polypeptide chains cross-linked at intervals forming long fibres or sheets.
Perform structural functional in cells and organisms e.g. collagen (tendons, bone, connective tissue), myosin (in muscle), fibroin silk (spiders’ webs), keratin (hair, horn, nails, feathers).
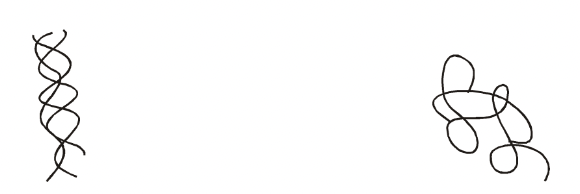
Fig. Fibrous Proteins Fig. Globular Proteins
Globular
Tertiary structure most important.
Polypeptide chains tightly folded to form spherical shape.
Easily soluble.
Form enzymes, antibodies and some hormones, e.g. Insulin. Other important roles.
Intermediate
Fibrous but soluble.
Forms insoluble fibrin when blood clots, e.g. fibrinogen.
2. According to Constitution
Conjugated Proteins
Name Prosthetic group Occurrence
Phosphoprotein Phosphoric acid Casein of milk, Vitellin of egg yolk
Glycoprotein Carbohydrate Membrane structure, Mucin (Component of saliva)
Nucleoprotein Nucleic acid Component of viruses, Chromosomes,
Ribosome structure
Chromoprotein Pigment Haemoglobin - haem (iron-containing pigment)
Phytochrome (plant pigment)
Cytochrome (respiratory pigment)
Lipoprotein Lipid Membrane structure
Lipid transported in blood as lipoprotein
Flavoprotein FAD (flavine adenine dinucleotide) Important in electron transport chain in respiration.
Metal proteins Metal E.g. nitrate reductase, the enzyme in plants which converts nitrate to nitrite
3. According to Function
Type Examples Occurrence/function
Structural Collagen, Keratin, Elastin Component of connective tissue, bone, tendons, cartilage. Skin feathers, nails, hair, horn.
Viral coat proteins Elastic connective tissue (ligaments); ‘Wraps up’ nucleic acid of virus
Enzymes Trypsin Catalyses hydrolysis of protein
Ribulose biphosphate Catalyses carboxylation (addition of (CO 2 ) to ribulose
carboxylase biphosphate in photosynthesis
Glutamine synthetase Catalyses synthesis of the amino acid glutamine from glutamic acid + ammonia
Hormones Insulin, Glucagon, ACTH Help to regulate glucose metabolism
Stimulates growth and activity of the adrenal cortex
Respiratory pigment Haemoglobin Transports O 2 in vertebrate blood
Myoglobin Stores O 2 in muscles
Transport Serum albumin Transport of fatty acids and lipids in blood
Protective Antibodies Forms complexes with foreign proteins
Fibrinogen Forms fibrin in blood clotting
Thrombin Involved in blood clotting mechanism
Contractile Myosin Moving filaments in myofibrils of muscle
Actin Stationary filaments in myofibrils of muscle
Storage Ovalbumin Egg white protein
Casein Milk protein
Toxins Snake venom Enzymes
Diphtheria toxin Toxin made by diphtheria bacteria
Made from amino acids and therefore always contain the element carbon, hydrogen, oxygen and nitrogen, and in some cases sulphur.
Some proteins form complexes with other molecules containing phosphorus, iron, zinc and copper. Macromolecules of high M, (relative formula mass or molecular mass between several thousands and several millions) consisting of chains of amino acids.
They are polymers and amino acids are the monomers. There are 20 different amino acids which are commonly found in naturally occurring proteins. The potential variety of proteins is unlimited because the sequence of amino acids in each protein is specific for that protein and is genetically controlled by the DNA of the cell in which it is made. Proteins are the most abundant organic molecules to be found in cells and form over 50% of their total dry mass. An essential component of the diet of animals and may be converted to both fat and carbohydrate by the cells. Their diversity enables them to display a great range of structural and metabolic activities within the organism.









