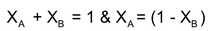Mole Concept
Some Basic Concept Of Chemistry of Class 11
Mole Concept
Atoms and molecules are too small to count. To solve this problem their numbers are expressed in terms of Avogadro’s number (NA = 6.023 × 1023). Mole is the number equal to Avogadro’s number just like a dozen is equal to 12, a century means 100, a score means = 20.
A mole (symbol mol) is defined as the amount of substance that contains as many atoms, molecules, ions electrons or any other elementary entities as there are carbon atoms in exactly 12 gm of 12 C . The number of atoms in 12 gm of 12 C is called Avogadro’s number (N A ).
N A = 6.023 x 10 23
One atomic mass unit (amu) = 1/N A = 1/6.023 x 10 23 gm = 1.66 x 10 -24 gm = 1.66 x 10 -27 kg
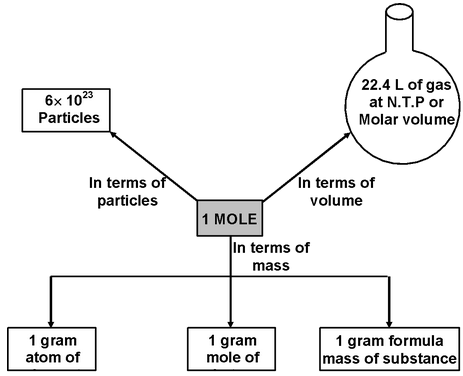
The number of moles of a substance can be calculated by various means depending on data available, as follows.
-
Number of moles of molecules
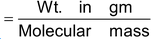
-
Number of moles of atoms
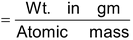
-
Number of moles of gases
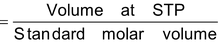 (Standard molar volume at STP = 22.4 lit)
(Standard molar volume at STP = 22.4 lit)
-
Number of moles of particles e.g. atoms, molecules ions etc

-

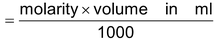
- for a compound Ax By, y moles of A = x moles of B
- Mole fraction = fraction of the substance in the mixture expressed in terms of mol is called its mol fraction (X)
E.g. for a mixture of substance A & B
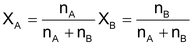 (n terms of denote number of moles)
(n terms of denote number of moles)