
Surface chemistry is the study of the phenomena on surfaces of substances. This applies to industries as well as everyday life.
Its application can be found in analytical work, medicine, the paint industry, etc. A surface chemistry study examines how chemical reactions occur at the interface of two surfaces: solids with liquids, solids with gases, solids with vacuums, liquids with gases, etc. Surface engineering refers to some of the applications of surface chemistry. Various phenomena occur on the surface of substances: Adsorption, Catalysis, Corrosion, Crystallization.
Adsorption
Adsorption is a surface phenomenon in which atoms, ions, or molecules of liquids, gases, or solids (dissolved) get accumulated at the surface of another substance (which is an indifferent phase) through adhesive forces. The substance that concentrates or accumulates at the surface of another substance is called adsorbate, and the material on which the adsorption takes place is called adsorbent.
Mechanism of Adsorption in Surface Chemistry
Adsorption occurs when molecules, atoms, or ions of one substance stick loosely to the surface of another substance.
It is an exothermic process, which means energy is liberated during this process. When one mole of the adsorbate is adsorbed on the adsorbent, the amount of heat that develops is called enthalpy. An increase in enthalpy is denoted as negative. This is due to the restriction of molecule freedom when adsorbate molecules are adsorbed onto a surface, resulting in a decrease in entropy. At constant pressure and temperature, adsorption can occur spontaneously.
Also Check : List of Chemistry Formulas
Types of Adsorption
According to the nature of the forces between the adsorbate and the adsorbent molecules, there are two types of adsorption:
Physical Adsorption
Adsorption in which weak van der Waals forces hold the adsorbate on the surface of the adsorbent is known as physical adsorption, or physisorption. Adsorption is non-specific and reversible. A gas's amount depends on its nature. A larger surface area allows for greater adsorption, so porous and finely divided metals are suitable for adsorption.
Characteristics of Adsorption
- There can be no specificity since any gas can get adsorbed onto the surface.
- Highly liquefiable gases are physically more adsorbent.
- An increase in pressure decreases the volume of gas and, therefore, increases gas molecule adsorption.
- When pressure decreases, gas molecules are removed from the solid surface, resulting in an exothermic adsorption process.
- Adsorption occurs readily at low temperatures and decreases as temperature increases (Le-Chatelier's principle).
- Due to their increased surface area, porous substances are better adsorbents.
- Activation does not require energy.
Download PDF Surface Chemistry Formula
Chemical Adsorption Chemisorption occurs when forces holding the adsorbate are as strong as chemical bonds.
Chemical Adsorption Characteristics:
- This are irreversible and definite.
- Gas adsorption does not depend on the critical temperature.
- As the surface area increases, it also increases.
- Similar to chemical bonds, there is a strong force of attraction.
- Layers formed by it are unimolecular.
- Adsorbent and adsorbate must form a chemical bond to exhibit the process-specific character.
- This is an exothermic process that increases the temperature.
- When the temperature is low and the pressure is high, it occurs slowly at low temperatures and more rapidly at higher temperatures.
Effects of Pressure and Temperature on Adsorption
Factors Affecting Adsorption of gas on Liquid
[caption id="attachment_14814" align="alignnone" width="300"]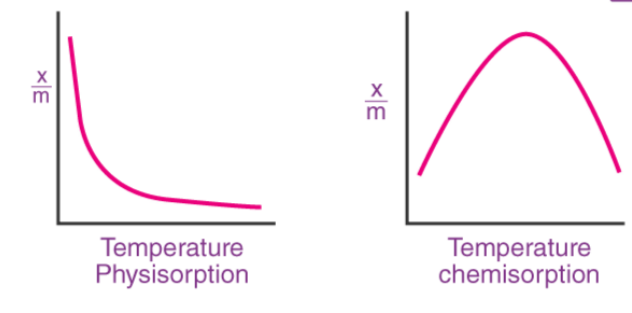 Effect of Temperature on Surface Chemistry[/caption]
Effect of Temperature on Surface Chemistry[/caption]
- At the same temperature, the same gas can be adsorbed by different solids to different extents. The surface area of the solid is directly proportional to the volume of the gas that has been adsorbed.
- Nature of the gas being adsorbed: Different gases are adsorbed to different extents even by the same solid. The reason is that as a gas's critical temperature increases, it becomes easier to liquefy and more readily adsorbed. This is because "the higher the temperature, the easier it is to liquefy”.
- The intermolecular forces of attraction are more effective on the adsorbent surface for a few gases, leading to greater adsorption.
Temperature :
Adsorption decreases with increasing temperature.
Pressure :
An increase in pressure enhances the adsorption of a gas at a constant temperature.
Freundlich Adsorption Isotherm:
German scientist Freundlich expressed an empirical relationship between the amount of gas adsorbed by the same mass of solid adsorbent and the pressure at a particular temperature in 1909. The equation goes as follows:
x/m = k.P 1/n (n > 1)
At a particular temperature, k and n are constants that depend on the nature of the adsorbent and the gas. 'x' is the gas's mass adsorbed on the adsorbent's mass at pressure 'P.'
To illustrate the relationship between pressure and adsorbent, one gram of the adsorbent is plotted as a curve. At a fixed pressure, physical adsorption decreases with temperature. At high pressure, the curves become saturated. Now, if you take the log of the equation above:
In this case, log x / m = log k + 1 / n log P
It is possible to examine the validity of the Freundlich isotherm by plotting log x/m on the y-axis and log P on the x-axis. If the plot represents a straight line, the Freundlich isotherm is valid. Otherwise, it is not.
A straight-line slope gives the value of 1/n, while an intercept on the y-axis indicates the importance of log k.
Limitations of Freundlich Isotherm:
The Freundlich Isotherm has the following limitations:
A Freundlich isotherm describes adsorption only approximately. 1/n lies between 0 and 1. Therefore, the equation holds true only at low pressures.
Adsorption is independent of pressure if 1/n = 0, x/m = 0.
Since 1/n = 1, x/m = k P, so x/m ∝ P, adsorption is directly proportional to pressure.
At high pressure, the experimental isotherms always reach saturation. Freundlich isotherm cannot explain this observation, and therefore fails at high pressure.
The Freundlich isotherm was followed by the Langmuir adsorption isotherm and the BET adsorption isotherm. The Langmuir isotherm assumed monolayer adsorption, whereas the BET isotherm assumed multilayer adsorption.
Applications of Adsorption
- A very high vacuum is created by the persistent traces of air adsorbed by charcoal from a vessel evacuated by a vacuum pump. An extremely high vacuum is obtained by combining a bundle of charcoal cooled in liquid air with a vessel that has already been vacuumed.
- It's an adsorbent system that uses activated charcoal or a mixture of adsorbents to adsorb poisonous gases in coal mines. It's usually worn to breath in coal mines. Adsorbents, such as activated charcoal or adsorbents, are used in coal mining to adsorb harmful gases (such as CO, Cl2 sulphur oxide, etc.) and purify the air.
- Moisture can be absorbed by silica gel and aluminium gel adsorbents.
- In order to remove colouring matter from solutions, follow these steps:
- In general, animal charcoal absorbs the colors of solutions by adsorbing coloured impurities.
- Solid catalysts facilitate a number of gaseous reactions of industrial importance due to the absorption of reactants on their solid surface. Heterogeneous catalysis reactions include the production of ammonia with iron as a catalyst, the development of H2SO4 with contact process, and the hydrogenation of oils with finely divided nickel.
- By adsorption on coconut charcoal at different temperatures, a mixture of noble gases is separated due to the different degree of gas adsorption.
- Adsorption of some drugs kills germs.
- A frothing agent and pine oil are used to separate low-grade sulphide ore from silica and other earthy matter.
- Surfaces of some sediments, such as silver halides (AgX), adsorb dyes such as eosin, fluorescein, etc., producing a characteristic color.
Catalysis
Reactants must surmount a certain amount of energy, called activation energy. While some reactant molecules possess enough kinetic energy to do this naturally, others don't. Hence, different reactions don't always happen at an equal rate under standard conditions. To address this, special reagents are added that reduce the necessary activation energy for converting reactants to products. These reagents are known as catalysts and catalysis is the process of reducing the activation energy.
- When heated, potassium chlorate decomposes slowly, releasing dioxygen. This decomposition occurs between 653 and 873 degrees Celsius.
2 KCl O 3 → 2 KCl + 3 O 2
As a result of MnO2, a much-accelerated decomposition occurs at 473 - 633 K. MnO2 acts as a catalyst.
2 KCl O 3 ——-→ 2 KCl + 3 O 2
Catalysts are substances that increase the reaction rate while remaining chemically and quantitatively unchanged after the reaction has taken place.
To promote NH3 by Haber's process, a promoter is a substance that increases the activity of a catalyst, while a poison is a substance that decreases the movement of a catalyst.
N 2 (g) + 3 H 2 (g)——2 N H 3 (g)
Catalysis of chemical reactions is divided into a few ways:
- Homogeneous Catalysis:
Homogeneous catalysis involves the reaction of reactants with the catalyst in the same phase, for example, hydrolysis of glucose in the presence of acid.
- Heterogeneous Catalysis:
The heterogeneous catalysis of chemical reactions occurs when the reactants involved in the reaction and the catalyst are in different phases.
- Positive catalysis:
Positive catalysts are foreign materials that accelerate a reaction. Below are some examples of positive catalysts.
In the presence of colloidal platinum, 2H2 decomposes.
2 H 2 O 2 (l) → 2 H 2 O 2 (l) + O 2 (g)
- Negative catalysis:
In the following examples, negative catalysts and inhibitors are used to slow down rather than speed up reactions.
If some alcohol is mixed with chloroform, the Oxidation by air is slowed.
2CH C l 2 (ℓ)+ O 2 (g)→2CO C l 2 (g)+2HCl(g) catalyst is Alcohol(ℓ)
- Photocatalysts:
Catalysis occurs when a catalyst receives light (such as visible light) and becomes excited.
Emulsion in Surface Chemistry
Emulsions are colloids where both the dispersion phase and dispersion medium are liquids. These liquids are immiscible or partially miscible; one is water.
Emulsion types:
In a nutshell, emulsions are colloidal solutions of two liquids that don't blend well, such as oil and water. To keep the emulsion stable, an emulsifying agent needs to be added; often materials like gum, soap or glass powder are used for this purpose.
There are two kinds of them:
Water dispersed with oil (O/W):
Milk and vanishing cream are dispersed in water. Water is dispersed in oil (w/o type), where oil serves as a dispersion medium.
As an example, butter and cream.
- For example, milk is an emulsion of soluble fats in water, in which oil acts as the dispersed phase (small amount) and water acts as the dispersed medium (excess).
- Casein and vanishing cream are emulsifiers, and these emulsions are referred to as aqueous emulsions.
- A water-in-oil emulsion has water as the dispersed phase and oil as the dispersion medium, for example, butter, cod liver oil, cold cream, and so on.
Surface Chemistry FAQs
How does surface chemistry work?
In surface chemistry, what are adsorption and absorption?
What is the Langmuir adsorption isotherm?
What is the impact of surface chemistry on catalysis?
In surface chemistry, what are surfactants?










