
Henry's Law Formula: Henry's Law is a fundamental principle in gas chemistry, stating that the amount of gas dissolved in a liquid is directly proportional to the partial pressure of that gas above the liquid, assuming a constant temperature. This relationship is characterized by a constant known as the Henry's Law constant, typically represented as 'kH.' The mathematical expression for Henry's Law can be expressed as follows:
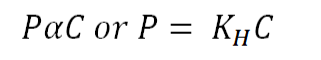
Here,
'P' represents the partial pressure of the gas in the atmosphere above the liquid.
'C' represents the concentration of the dissolved gas.
'kH' stands for the Henry's Law constant specific to the gas in question.
The origins of this law trace back to the early 19th century when it was established by the English chemist William Henry. It's worth noting that the Henry's law constant can be represented in two distinct manners. When expressed in relation to solubility and pressure, it is termed the Henry's law solubility constant, typically denoted as 'H.' Conversely, when the constant is defined in relation to pressure and solubility, it is referred to as the Henry's law volatility constant, typically represented as 'kH.'
Also Check – Vapor Pressure Formula
Illustration of Henry's Law Relationship
The diagram above provides a visual representation of the connection between the solubility of a gas in a liquid and the partial pressure of that gas in the atmosphere above the liquid, as described by Henry's law. It's important to note that a higher partial pressure of the gas results in greater solubility in the liquid.
Examples Illustrating Henry's Law
Henry's law is evident in various everyday scenarios, such as when opening a bottle of Pepsi or any other carbonated beverage. Before opening, the gas above the sealed carbonated drink primarily consists of pure carbon dioxide, maintained at a pressure slightly higher than standard atmospheric pressure. Henry's law dictates that, as a consequence, the carbon dioxide has high solubility in the unopened drink.
Upon opening the bottle, pressurized CO2 escapes into the atmosphere, often accompanied by a distinctive hissing sound. As the partial pressure of CO2 in the atmosphere above the drink rapidly decreases, the solubility of carbon dioxide in the liquid also decreases, in accordance with Henry's law. This causes the dissolved CO2 to form tiny bubbles and rise to the surface of the drink, eventually escaping into the atmosphere.
If the carbonated drink remains open for an extended period, the concentration of carbon dioxide in the liquid will eventually reach equilibrium with the concentration of carbon dioxide in the atmosphere (approximately 0.05%). This equilibrium results in the drink losing its 'fizzy' taste and going flat.
Also Check – Gibbs Free Energy Formula
Respiration and Oxygenation of Blood
During the respiration process, inhalation results in an elevation of the partial pressure of oxygen within the alveoli. When deoxygenated blood encounters the oxygen-rich air within the alveoli, the following gas exchange events occur due to the principles of Henry's law:
Given the high partial pressure of oxygen in the alveoli and the low level of dissolved oxygen in deoxygenated blood, oxygen naturally moves from the alveoli into the bloodstream.
The alveoli maintain a very low partial pressure of carbon dioxide (with CO2 making up only about 0.05% of the atmosphere), while the concentration of dissolved CO2 in deoxygenated blood is notably high. Consequently, carbon dioxide migrates from the blood into the alveoli. This carbon dioxide is subsequently expelled from the body through exhalation.
In this manner, Henry's law assumes a pivotal role in the respiration processes of various organisms.
Also Check – Vapor Pressure Formula
Factors Affecting the Henry’s Law Constant
The Henry's law constant for a gas is influenced by several key factors, including:
- The specific gas in question.
- The properties of the solvent.
- Temperature and pressure conditions.
As a result, distinct gases exhibit varying Henry's law constants when dissolved in different solvents, as depicted graphically below.
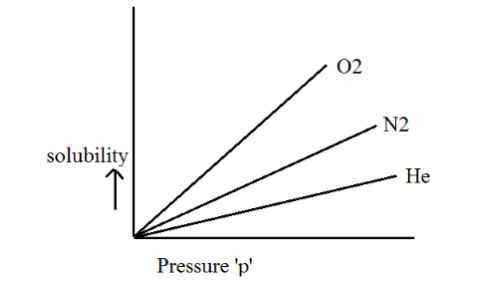
Limitations of Henry’s Law
- Henry’s law applies solely in situations where the system's molecules are in an equilibrium state.
- At extremely elevated pressures, Henry’s law no longer holds true for gases.
- When the gas and the solution engage in chemical reactions, the law does not apply.
Also Check – Dilution Formula
Henry’s Law Formula Solved Examples
Example 1: Determine the solubility of nitrogen gas (N2) in water at a temperature of 298 K when the partial pressure of N2 is 0.5 atm. Given that the Henry's law constant (kH) for N2 is 6.5*10-4 mol/(L·atm).
Using Henry's law equation, P = kH * C:
C = P / kH = 0.5 atm / (6.5*10-4 mol/(L·atm)) ≈ 769.23 M
So, the solubility of nitrogen gas in water at the given conditions is approximately 769.23 M.
Example 2 : Calculate the partial pressure of hydrogen gas (H2) required to achieve a solubility of 1.5*10-3 M in water at 25°C. The Henry's law constant (kH) for H2 at this temperature is 7.8104 atm·L/mol.
Using Henry's law:
P = kH * C = (7.8104 atm·L/mol) * (1.5*10-3 mol/L) = 117 atm
Thus, a partial pressure of 117 atm of hydrogen gas is needed to achieve a solubility of 1.5*10-3 M in water at 25°C.
What is Henry's Law?
What is the Henry's Law constant (kH)?
What happens to gas solubility when pressure increases according to Henry's Law?
How does Henry's Law apply to everyday life?










